Professional Documents
Culture Documents
Chemical Kinetics-4
Chemical Kinetics-4
Uploaded by
IshikaCopyright:
Available Formats
You might also like
- Batch Reactor Exp.Document21 pagesBatch Reactor Exp.Laila Al-shafieNo ratings yet
- Week 2 Chemical Kinetics Analysis of Rate EquationDocument31 pagesWeek 2 Chemical Kinetics Analysis of Rate EquationPeachYpeachasNo ratings yet
- Resistance Vs Temperature Experiment Lab ReportDocument7 pagesResistance Vs Temperature Experiment Lab ReportEmily Gatlin67% (3)
- Activation EnergyDocument3 pagesActivation EnergyAditya ShanmukhaNo ratings yet
- Factors Influencing Rate of Reaction (R.U.Birajdar)Document12 pagesFactors Influencing Rate of Reaction (R.U.Birajdar)RayNo ratings yet
- 20D. The Arrhenius EquationDocument19 pages20D. The Arrhenius EquationhayyiratulfatimahNo ratings yet
- Lecture - 16-Enzyme Kinetics and Catalysis 1Document36 pagesLecture - 16-Enzyme Kinetics and Catalysis 1Nagarjuna VuchuruNo ratings yet
- Activation Energy: - The Arrhenius EquationDocument19 pagesActivation Energy: - The Arrhenius EquationemilyNo ratings yet
- Chemical Kinetics 28072022Document19 pagesChemical Kinetics 28072022Kartik IyerNo ratings yet
- Activation Energy2Document27 pagesActivation Energy2Chen ShyanNo ratings yet
- 3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesDocument9 pages3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesAiman ButtNo ratings yet
- Reaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksDocument12 pagesReaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksIAS IndiaNo ratings yet
- ElectrochemistryDocument6 pagesElectrochemistryGovind ManglaniNo ratings yet
- Lecture 9 Evans DiagramsDocument33 pagesLecture 9 Evans DiagramsDaniel UlloaNo ratings yet
- Lakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Document3 pagesLakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Meet ShahNo ratings yet
- Transition State With Catalyst G Activation Free Energy G Driving Force GDocument10 pagesTransition State With Catalyst G Activation Free Energy G Driving Force GMir RafsanNo ratings yet
- Gibbs Free Energy and Chemical Equilibrium: Department of Chemistry IPB UniversityDocument56 pagesGibbs Free Energy and Chemical Equilibrium: Department of Chemistry IPB UniversityAna Sholikhatus Sa'diyahNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Administracion OTIC IVICNo ratings yet
- PdfjoinerDocument47 pagesPdfjoinermdilshadshigri1000No ratings yet
- 11.3 Factors Affecting Reaction RateDocument23 pages11.3 Factors Affecting Reaction RateAvicenna Ibnu BahrinNo ratings yet
- N Calculatio of Basis As A Reactant Limiting Choose D D C C B B A ADocument13 pagesN Calculatio of Basis As A Reactant Limiting Choose D D C C B B A AsehunNo ratings yet
- Classical Theory of Heat Capacity of SolidsDocument19 pagesClassical Theory of Heat Capacity of SolidsPrateek DasNo ratings yet
- Topic 2: Potentiometric Methods of AnalysisDocument19 pagesTopic 2: Potentiometric Methods of AnalysisYting TanNo ratings yet
- Typically Rates of Reactions Double For Every 10 C Rise in TemperatureDocument22 pagesTypically Rates of Reactions Double For Every 10 C Rise in TemperatureZainab HasanNo ratings yet
- Chap8 2Document17 pagesChap8 2Usman BlembengNo ratings yet
- Note The Increase in The Shelf Life of Suspensions This Is Because TheDocument12 pagesNote The Increase in The Shelf Life of Suspensions This Is Because TheValentina Medina MoralesNo ratings yet
- 07AMSCH23 - Physical Chemistry - IIDocument260 pages07AMSCH23 - Physical Chemistry - IIALPHY100% (1)
- Chapter 1 PBLMDocument2 pagesChapter 1 PBLMSOPHIYA GNo ratings yet
- Chapter 2Document42 pagesChapter 2MariaAlejandraReyesNo ratings yet
- Electrochemical Kinetics - Basics PDFDocument9 pagesElectrochemical Kinetics - Basics PDFJúlio Gabriel Queiroz dos SantosNo ratings yet
- CHE 102 Formula SheetsDocument3 pagesCHE 102 Formula SheetsMarjuk MahfuzNo ratings yet
- Arrehnius QuestionsDocument3 pagesArrehnius QuestionsJolNo ratings yet
- Allen 1966Document2 pagesAllen 1966SouravNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsShubhankar SinhaNo ratings yet
- Chap 52 Activation EnergyDocument7 pagesChap 52 Activation Energybreakfast noNo ratings yet
- Kinetika Kimia Pada Laju ReaksiDocument25 pagesKinetika Kimia Pada Laju ReaksiWardahNo ratings yet
- Physical Chemistry: Theoretical Chemical KineticsDocument18 pagesPhysical Chemistry: Theoretical Chemical Kineticsjeep2014No ratings yet
- Energy of ActivationDocument10 pagesEnergy of ActivationAditya VermaNo ratings yet
- Ps2 in PDCDocument3 pagesPs2 in PDClily august0% (1)
- Atoms in Complex Twisted Light: Journal of OpticsDocument68 pagesAtoms in Complex Twisted Light: Journal of Opticsfabian correaNo ratings yet
- NoteDocument7 pagesNoteRosina KaneNo ratings yet
- Week 6Document4 pagesWeek 6vivaline AchiengNo ratings yet
- Effect of Temperature On The Rate Constant-Arrhenius EquationDocument8 pagesEffect of Temperature On The Rate Constant-Arrhenius Equationwinifred ekpoNo ratings yet
- Chemistry Notes Electrochemistry NotesDocument5 pagesChemistry Notes Electrochemistry Notesguna100% (1)
- Chemical KineticsDocument2 pagesChemical KineticsLuluNo ratings yet
- Molecular KineticsDocument6 pagesMolecular Kineticssonia.morell.obNo ratings yet
- I: Lasers and Optical FibersDocument41 pagesI: Lasers and Optical FibersVaibhavNo ratings yet
- Electronic Properties of Materials: Bonding and Structure of MoleculesDocument57 pagesElectronic Properties of Materials: Bonding and Structure of Moleculespham minhNo ratings yet
- ElectrochemistryDocument40 pagesElectrochemistryՏɑʍ ՏɑղԵհօՏհNo ratings yet
- Electrochemical SystemDocument19 pagesElectrochemical SystemJim tanNo ratings yet
- TME 304unit 2 - LEC 5Document8 pagesTME 304unit 2 - LEC 5BoweNo ratings yet
- Reaction KineticsDocument26 pagesReaction Kineticssuhaila ramleyNo ratings yet
- Chapter 3 Part 1Document23 pagesChapter 3 Part 1toomas.ijimNo ratings yet
- Computational ChemistryDocument16 pagesComputational ChemistryTaimoor Hassan KhanNo ratings yet
- Differential RelayDocument9 pagesDifferential RelaysourabhNo ratings yet
- CHEM. 204: Kinetic Properties of Chemical ReactionsDocument7 pagesCHEM. 204: Kinetic Properties of Chemical ReactionsmyriamNo ratings yet
- Chapter 4. Effect of Kinetic Factors On Reaction RatesDocument18 pagesChapter 4. Effect of Kinetic Factors On Reaction RatesTrucNo ratings yet
- Kinetics Basic Elements VDocument8 pagesKinetics Basic Elements Vbibeka Computer123No ratings yet
- Molecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryFrom EverandMolecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryNo ratings yet
Chemical Kinetics-4
Chemical Kinetics-4
Uploaded by
IshikaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Kinetics-4
Chemical Kinetics-4
Uploaded by
IshikaCopyright:
Available Formats
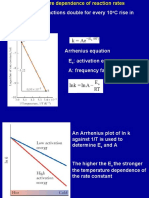
2 Arrhenius approach of temperature dependence on rate
of reaction
8 f conch K
attested
temperature
on changing temperature catalyst
K changes and hence the surface area
rate of reaction changes intensityof light
Arrhenius equation
K AéÉ
A Arrhenius factor frequency factor pre exponentialfactor
Ea activation energy
A Ea f T
A Ea f nature of reaction
considering reaction
Az g t B2 gl 2 AB g
A B B A B
A B A B
Intermediate
ace to reaction can take place when a molecule
Arrhenius
of Az collides with a molecule of B to form an unstable
intermediate which exists for very short time and then
breaks up to form 2 molecules of AB
The energy required to
form intermediate
called activatedcomplex
is known as activation
Estads negated energy Ea
with the help of Maxwell's speed distribution curve
K AéÉ
fraction of molecules having
kinetic energy Ea
taking log
Ink 1nA
logk log 2 383k
logk log A 2.58321
log II 2.3 32
log Kz log A z 5583122
Graphicalrepresentation of Arrhenius equation
T
generally
You might also like
- Batch Reactor Exp.Document21 pagesBatch Reactor Exp.Laila Al-shafieNo ratings yet
- Week 2 Chemical Kinetics Analysis of Rate EquationDocument31 pagesWeek 2 Chemical Kinetics Analysis of Rate EquationPeachYpeachasNo ratings yet
- Resistance Vs Temperature Experiment Lab ReportDocument7 pagesResistance Vs Temperature Experiment Lab ReportEmily Gatlin67% (3)
- Activation EnergyDocument3 pagesActivation EnergyAditya ShanmukhaNo ratings yet
- Factors Influencing Rate of Reaction (R.U.Birajdar)Document12 pagesFactors Influencing Rate of Reaction (R.U.Birajdar)RayNo ratings yet
- 20D. The Arrhenius EquationDocument19 pages20D. The Arrhenius EquationhayyiratulfatimahNo ratings yet
- Lecture - 16-Enzyme Kinetics and Catalysis 1Document36 pagesLecture - 16-Enzyme Kinetics and Catalysis 1Nagarjuna VuchuruNo ratings yet
- Activation Energy: - The Arrhenius EquationDocument19 pagesActivation Energy: - The Arrhenius EquationemilyNo ratings yet
- Chemical Kinetics 28072022Document19 pagesChemical Kinetics 28072022Kartik IyerNo ratings yet
- Activation Energy2Document27 pagesActivation Energy2Chen ShyanNo ratings yet
- 3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesDocument9 pages3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesAiman ButtNo ratings yet
- Reaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksDocument12 pagesReaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksIAS IndiaNo ratings yet
- ElectrochemistryDocument6 pagesElectrochemistryGovind ManglaniNo ratings yet
- Lecture 9 Evans DiagramsDocument33 pagesLecture 9 Evans DiagramsDaniel UlloaNo ratings yet
- Lakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Document3 pagesLakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Meet ShahNo ratings yet
- Transition State With Catalyst G Activation Free Energy G Driving Force GDocument10 pagesTransition State With Catalyst G Activation Free Energy G Driving Force GMir RafsanNo ratings yet
- Gibbs Free Energy and Chemical Equilibrium: Department of Chemistry IPB UniversityDocument56 pagesGibbs Free Energy and Chemical Equilibrium: Department of Chemistry IPB UniversityAna Sholikhatus Sa'diyahNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Administracion OTIC IVICNo ratings yet
- PdfjoinerDocument47 pagesPdfjoinermdilshadshigri1000No ratings yet
- 11.3 Factors Affecting Reaction RateDocument23 pages11.3 Factors Affecting Reaction RateAvicenna Ibnu BahrinNo ratings yet
- N Calculatio of Basis As A Reactant Limiting Choose D D C C B B A ADocument13 pagesN Calculatio of Basis As A Reactant Limiting Choose D D C C B B A AsehunNo ratings yet
- Classical Theory of Heat Capacity of SolidsDocument19 pagesClassical Theory of Heat Capacity of SolidsPrateek DasNo ratings yet
- Topic 2: Potentiometric Methods of AnalysisDocument19 pagesTopic 2: Potentiometric Methods of AnalysisYting TanNo ratings yet
- Typically Rates of Reactions Double For Every 10 C Rise in TemperatureDocument22 pagesTypically Rates of Reactions Double For Every 10 C Rise in TemperatureZainab HasanNo ratings yet
- Chap8 2Document17 pagesChap8 2Usman BlembengNo ratings yet
- Note The Increase in The Shelf Life of Suspensions This Is Because TheDocument12 pagesNote The Increase in The Shelf Life of Suspensions This Is Because TheValentina Medina MoralesNo ratings yet
- 07AMSCH23 - Physical Chemistry - IIDocument260 pages07AMSCH23 - Physical Chemistry - IIALPHY100% (1)
- Chapter 1 PBLMDocument2 pagesChapter 1 PBLMSOPHIYA GNo ratings yet
- Chapter 2Document42 pagesChapter 2MariaAlejandraReyesNo ratings yet
- Electrochemical Kinetics - Basics PDFDocument9 pagesElectrochemical Kinetics - Basics PDFJúlio Gabriel Queiroz dos SantosNo ratings yet
- CHE 102 Formula SheetsDocument3 pagesCHE 102 Formula SheetsMarjuk MahfuzNo ratings yet
- Arrehnius QuestionsDocument3 pagesArrehnius QuestionsJolNo ratings yet
- Allen 1966Document2 pagesAllen 1966SouravNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsShubhankar SinhaNo ratings yet
- Chap 52 Activation EnergyDocument7 pagesChap 52 Activation Energybreakfast noNo ratings yet
- Kinetika Kimia Pada Laju ReaksiDocument25 pagesKinetika Kimia Pada Laju ReaksiWardahNo ratings yet
- Physical Chemistry: Theoretical Chemical KineticsDocument18 pagesPhysical Chemistry: Theoretical Chemical Kineticsjeep2014No ratings yet
- Energy of ActivationDocument10 pagesEnergy of ActivationAditya VermaNo ratings yet
- Ps2 in PDCDocument3 pagesPs2 in PDClily august0% (1)
- Atoms in Complex Twisted Light: Journal of OpticsDocument68 pagesAtoms in Complex Twisted Light: Journal of Opticsfabian correaNo ratings yet
- NoteDocument7 pagesNoteRosina KaneNo ratings yet
- Week 6Document4 pagesWeek 6vivaline AchiengNo ratings yet
- Effect of Temperature On The Rate Constant-Arrhenius EquationDocument8 pagesEffect of Temperature On The Rate Constant-Arrhenius Equationwinifred ekpoNo ratings yet
- Chemistry Notes Electrochemistry NotesDocument5 pagesChemistry Notes Electrochemistry Notesguna100% (1)
- Chemical KineticsDocument2 pagesChemical KineticsLuluNo ratings yet
- Molecular KineticsDocument6 pagesMolecular Kineticssonia.morell.obNo ratings yet
- I: Lasers and Optical FibersDocument41 pagesI: Lasers and Optical FibersVaibhavNo ratings yet
- Electronic Properties of Materials: Bonding and Structure of MoleculesDocument57 pagesElectronic Properties of Materials: Bonding and Structure of Moleculespham minhNo ratings yet
- ElectrochemistryDocument40 pagesElectrochemistryՏɑʍ ՏɑղԵհօՏհNo ratings yet
- Electrochemical SystemDocument19 pagesElectrochemical SystemJim tanNo ratings yet
- TME 304unit 2 - LEC 5Document8 pagesTME 304unit 2 - LEC 5BoweNo ratings yet
- Reaction KineticsDocument26 pagesReaction Kineticssuhaila ramleyNo ratings yet
- Chapter 3 Part 1Document23 pagesChapter 3 Part 1toomas.ijimNo ratings yet
- Computational ChemistryDocument16 pagesComputational ChemistryTaimoor Hassan KhanNo ratings yet
- Differential RelayDocument9 pagesDifferential RelaysourabhNo ratings yet
- CHEM. 204: Kinetic Properties of Chemical ReactionsDocument7 pagesCHEM. 204: Kinetic Properties of Chemical ReactionsmyriamNo ratings yet
- Chapter 4. Effect of Kinetic Factors On Reaction RatesDocument18 pagesChapter 4. Effect of Kinetic Factors On Reaction RatesTrucNo ratings yet
- Kinetics Basic Elements VDocument8 pagesKinetics Basic Elements Vbibeka Computer123No ratings yet
- Molecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryFrom EverandMolecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryNo ratings yet