Professional Documents
Culture Documents
Lecture - TCA Cycle and The Electron Transport Chain
Lecture - TCA Cycle and The Electron Transport Chain
Uploaded by
aatrrisdal20260 ratings0% found this document useful (0 votes)
6 views2 pagesOriginal Title
Lecture- TCA cycle and the Electron Transport Chain
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
6 views2 pagesLecture - TCA Cycle and The Electron Transport Chain
Lecture - TCA Cycle and The Electron Transport Chain
Uploaded by
aatrrisdal2026Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
● Amino acids, sugars, and lipids feed into the TCA cycle
● The TCA products are as follows:
○ Carbon dioxide
○ NADH
○ FADH2
○ ATP
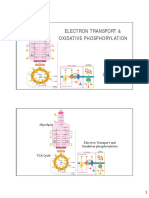
The Electron Transport Chain
● The goal of the electron transport chain is to use NADH and FADH2 to concentrate H+
protons in the intermembrane space
● Electrochemical potential is generated across this membrane in the mitochondria- Inner
mitochondrial membrane
● Electron transport across the inner mitochondrial membrane creates a proton gradient
which is used to fuel ATP synthesis
● The energy provided by the oxidation of NADH into NAD+
○ Instead of ATP, the ETC uses electrons from NADH or FADH2
○ The hydride ion is removed from NADH (to regenerate NAD+) and is converted
into a proton and two electrons (H-→ H+ + 2e -)
● The ETC converts NADH reduction into a gradient of protons
○ The electrons start with very high energy and gradually lose it as they pass along
the chain.
○ Electrons pass sequentially from one complex to another until they are finally
transferred to oxygen
● The ETC requires the close association of the electron carriers with protein
molecules.The electron carriers are prosthetic groups
○ Prosthetic groups are large non-protein molecules embedded in the protein
○ Complicated chemical structures not possible with amino acids
○ Flavin mononucleotide (comes from Vitamin B2)
● Example: Heme is the prosthetic group in hemoglobin and is necessary for oxygen
binding
● Complex I receives 2 electrons from NADH and passes them to CoQ. The energy is
used to pump 4 H+
● Coenzyme Q (CoQ) is a lipid-like carrier (aka ubiquinone)
● Coenzyme Q is also known as Succinate dehydrogenase
● Complex II receives 2 electrons from succinate passing them directly to FADH2 and then
into CoQ. Does not pump H+.
● Complex III receives 2 electrons from CoQ and passes them to Cytochrome C. The
energy is used to pump 4 H+
● Cytochrome c is a small protein that serves as carrier of electrons
○ CytC moves Electrons from complex 3 to 4
○ CytC is a small protein with a Heme cofactor
○ Heme is the electron carrier
● Complex IV receives 2 electrons from Cytochrome C and passes them to Oxygen, which
is reduced to water. The energy is used to pump 2 H+
● Electrons move in a single direction because Redox centers (electron carriers) are
organized from low to high affinity
● The redox centers and the H+ pumps are separated in complex I (Electron transport and
Tandem Proton pumping)
● Structural changes in Complex I direct H+ to move through translocation half-channels
○ Half-channels are formed by conserved polar residues and polar cavities
containing water molecules
● Structural changes in Complex I direct H+ to move through translocation half-channels
● The ETC forms supramolecular assemblies or supercomplexes- meaning that the
complexes are clustered together
● Finally, the H+ flow back powering the synthesis of ATP
○ The power source for the ATP synthase is a difference in the concentration of H+
on opposite sides of the inner mitochondrial membrane.
○ Protons flow down their concentration gradient into the matrix through the
membrane protein ATP synthase, causing it to spin
○ ATP synthesis takes place in the ATP synthase complex of the Electron transport
chain, which is found in the inner mitochondrial membrane. It is fuelled by the
proton gradient created by oxidation of high energy molecules such as NADH
and FADH2.
You might also like
- Test Bank For Campbell Essential Biology 7th by SimonDocument36 pagesTest Bank For Campbell Essential Biology 7th by Simonfuturityerlkingfd6d100% (33)
- CH 14 Inheritance Student1Document15 pagesCH 14 Inheritance Student1Tajul Azhar BaharudinNo ratings yet
- 6 Electron Transport Chain NoteDocument21 pages6 Electron Transport Chain Notevgf58jsq6cNo ratings yet
- Respiratory Chain & Oxidative PhosphorylationDocument57 pagesRespiratory Chain & Oxidative PhosphorylationHanifa AffianiNo ratings yet
- Electron Transport ChainDocument4 pagesElectron Transport ChainBae SeujiNo ratings yet
- Electron Transport SystemDocument58 pagesElectron Transport SystemSantosh KumarNo ratings yet
- Electron Transport and Oxidative Phosphorylation: Refer To: Lehninger Principles of Biochemistry (Chapter 19)Document43 pagesElectron Transport and Oxidative Phosphorylation: Refer To: Lehninger Principles of Biochemistry (Chapter 19)Yousef KhallafNo ratings yet
- Bioenergetics Part 3Document36 pagesBioenergetics Part 3CM Nursing DepartmentNo ratings yet
- Conversion of Food Into EnergyDocument65 pagesConversion of Food Into EnergyFeddanie CapiliNo ratings yet
- 1 Oxidative PhosphorylationDocument10 pages1 Oxidative PhosphorylationRoland ToroNo ratings yet
- AtthapuDocument21 pagesAtthapuPrashanth PuttapagaNo ratings yet
- Electron Transport ChainDocument7 pagesElectron Transport ChainMaria HarisNo ratings yet
- ChemiosmosisDocument7 pagesChemiosmosisSkenzKenzNo ratings yet
- ETC NotesDocument3 pagesETC Notesaminahali04.aaNo ratings yet
- The Electron Transport System Also Called The Electron Transport ChainDocument5 pagesThe Electron Transport System Also Called The Electron Transport ChainRe UpNo ratings yet
- Title: Electron Transport System (Aerobic)Document4 pagesTitle: Electron Transport System (Aerobic)GISRemoteSensingNo ratings yet
- Electron Transport ChainDocument3 pagesElectron Transport ChainEmma MelNo ratings yet
- Electron Transport Chain BSBT025F18 PDFDocument7 pagesElectron Transport Chain BSBT025F18 PDFZaki SyedNo ratings yet
- Electron Transport and Chemiosmosis-5thDocument68 pagesElectron Transport and Chemiosmosis-5tholawandeilo123No ratings yet
- Electron Transport Chain PPT 5Document39 pagesElectron Transport Chain PPT 5rohajira67% (3)
- السلسلة التنفسيةDocument14 pagesالسلسلة التنفسيةامجد حسين جواد كاظمNo ratings yet
- Oxidative Phosphorylation and Electron Transport Chain II Respiratory Complexes Structure and Organization Chemiosmotic TheoryDocument20 pagesOxidative Phosphorylation and Electron Transport Chain II Respiratory Complexes Structure and Organization Chemiosmotic TheoryIffatnazNo ratings yet
- Oxidative Phosphorylation-WPS OfficeDocument30 pagesOxidative Phosphorylation-WPS OfficeZoyaNo ratings yet
- Cells and Sugars 09 Mitochondria and Ox Phos StudentDocument21 pagesCells and Sugars 09 Mitochondria and Ox Phos StudenttyhbbhhNo ratings yet
- Chapter 20 ETC and Oxidative PhosphDocument29 pagesChapter 20 ETC and Oxidative PhosphSpencer ThomasNo ratings yet
- Electron Transport ChainDocument18 pagesElectron Transport ChainArlyn Pasion BungbongaNo ratings yet
- Electron Transport Chain ExplainedDocument9 pagesElectron Transport Chain Explainedmaria genioNo ratings yet
- TCA CycleDocument22 pagesTCA CycleEMMANUEL ABEL IMAHNo ratings yet
- Electron TransportDocument17 pagesElectron TransportKofi Fofie-AsieduNo ratings yet
- 2021 Learning Material in MC 2 Chapter 7Document5 pages2021 Learning Material in MC 2 Chapter 7Domusthones TuplanoNo ratings yet
- 20 Electron TransportDocument41 pages20 Electron TransportGian BanaresNo ratings yet
- Fosforilasi OksidatifDocument82 pagesFosforilasi OksidatifSanti WilujengNo ratings yet
- Etc (Electron Transport Chain)Document24 pagesEtc (Electron Transport Chain)Dark_KiroNo ratings yet
- Lehninger Principles of Biochemistry PDFDocument37 pagesLehninger Principles of Biochemistry PDFmonitamiftah100% (1)
- Electron Transport ChainDocument33 pagesElectron Transport ChainAfaq AhmadNo ratings yet
- Electron Transport ch14Document21 pagesElectron Transport ch14tania.delafuenteNo ratings yet
- MetabolicBiochemistry Sem1 2021 Lecture6 Part2Document30 pagesMetabolicBiochemistry Sem1 2021 Lecture6 Part2kristal eliasNo ratings yet
- Oxidation Glucose Coenzymes Glycolysis Citric Acid Cycle: Electron TransportDocument4 pagesOxidation Glucose Coenzymes Glycolysis Citric Acid Cycle: Electron TransportHabibur RahamanNo ratings yet
- BiochemDocument10 pagesBiochemHoàng LâmNo ratings yet
- Electron Transport ChainetcDocument19 pagesElectron Transport Chainetcpk kaleenaNo ratings yet
- Electron Transport Chain andDocument25 pagesElectron Transport Chain andLovely Joy Aranda CurammengNo ratings yet
- Etc N Oxid PhoshphorylationDocument74 pagesEtc N Oxid Phoshphorylationhassanainshahi13No ratings yet
- Etc PDFDocument14 pagesEtc PDFjamalNo ratings yet
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocument27 pagesOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreYoAmoNYC100% (1)
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocument27 pagesOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorNo ratings yet
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocument27 pagesOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreJSlinkNYNo ratings yet
- Electron Transport ChainDocument15 pagesElectron Transport Chainvanshdeep SinghNo ratings yet
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocument27 pagesOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorNo ratings yet
- Cellular RespirationDocument55 pagesCellular RespirationFuad sabsebNo ratings yet
- Bioemergetics Cellular RespDocument59 pagesBioemergetics Cellular RespHassan Abib BasalNo ratings yet
- Electron Transport ChainDocument5 pagesElectron Transport ChainTanya Dilshad100% (2)
- Electron Transport Chain - WikipediaDocument53 pagesElectron Transport Chain - WikipediaLsaurusNo ratings yet
- ETCDocument8 pagesETCRy L.No ratings yet
- Electron Transport and Oxidative PhosphorylationDocument38 pagesElectron Transport and Oxidative PhosphorylationSyedaNaveenBatoolNo ratings yet
- Cellular Energy TransactionsDocument3 pagesCellular Energy TransactionsDrAmit VermaNo ratings yet
- Electron Transport Chai1Document6 pagesElectron Transport Chai1shanto.tn98No ratings yet
- ASSIGNMENT NO2 ChemDocument5 pagesASSIGNMENT NO2 ChemD AmanatNo ratings yet
- Lecture 6: Part 1 Oxidative Phosphorylation and ETC: Produced by Samira Aili Presented by Alvaro GarciaDocument23 pagesLecture 6: Part 1 Oxidative Phosphorylation and ETC: Produced by Samira Aili Presented by Alvaro Garciakristal elias100% (1)
- Oxidative PhosphorylationDocument18 pagesOxidative Phosphorylationjason misticaNo ratings yet
- Etc 180815173359Document17 pagesEtc 180815173359jyothi sai sriNo ratings yet
- Electron Transport and Oxidative Phosphorylation: Paul D. Adams - University of ArkansasDocument43 pagesElectron Transport and Oxidative Phosphorylation: Paul D. Adams - University of ArkansasKrishly Ann Medina SalazarNo ratings yet
- Evidence of EvolutionDocument19 pagesEvidence of EvolutionOmar FarooqNo ratings yet
- Bi 328 - Comparative Vertebrate Anatomy: Biology 328 - Winter 2016Document3 pagesBi 328 - Comparative Vertebrate Anatomy: Biology 328 - Winter 2016Asad AsadNo ratings yet
- Microbiology SyllabusDocument2 pagesMicrobiology Syllabusapi-3704804No ratings yet
- Contoh Soal Science PrimaryDocument5 pagesContoh Soal Science PrimaryErnazNo ratings yet
- IB 204 SyllabusDocument7 pagesIB 204 SyllabusQuân VōNo ratings yet
- Structure and Functions of Ti and Ri PlasmidsDocument3 pagesStructure and Functions of Ti and Ri PlasmidsSasikumar SankarNo ratings yet
- Physiological Basis of BehaviorDocument35 pagesPhysiological Basis of BehaviorAnna Rhea BurerosNo ratings yet
- Biological Science DepartmentDocument8 pagesBiological Science DepartmentInga Budadoy NaudadongNo ratings yet
- Entry-Level Biochemist Resume Sample - MonsterDocument3 pagesEntry-Level Biochemist Resume Sample - MonsterxydiaNo ratings yet
- Full Chapter Clinical Atlas of Small Animal Cytology and Hematology 2Nd Edition Burton PDFDocument53 pagesFull Chapter Clinical Atlas of Small Animal Cytology and Hematology 2Nd Edition Burton PDFhubert.prat414100% (5)
- Test-32 With KeyDocument29 pagesTest-32 With KeyIllahi Bakhsh MariNo ratings yet
- 8 - geneMAP™ Thrombophilia Panel V2.3Document9 pages8 - geneMAP™ Thrombophilia Panel V2.3mirian flechaNo ratings yet
- Human Genome ProjectDocument22 pagesHuman Genome Projecttani84638No ratings yet
- Cell Cycle and MitosisDocument26 pagesCell Cycle and MitosisEngieluz Fontillas LptNo ratings yet
- Jawapan Bio-Score Bab 1 (Form 5)Document45 pagesJawapan Bio-Score Bab 1 (Form 5)azamsensei94% (32)
- Critical Thinking: Dialysis MachineDocument19 pagesCritical Thinking: Dialysis MachinerushikantNo ratings yet
- 1 3 3 Evidence ReportDocument11 pages1 3 3 Evidence Reportapi-296590894No ratings yet
- Ethical Issues in Cloning.Document20 pagesEthical Issues in Cloning.Neelesh Bhandari100% (1)
- Stem Cell-Based Therapies For Parkinson DiseaseDocument17 pagesStem Cell-Based Therapies For Parkinson DiseaseYunita Christiani BiyangNo ratings yet
- Principles of Cytocentrifugation: CytologyDocument4 pagesPrinciples of Cytocentrifugation: CytologyNAKANWAGI JOSLYLINENo ratings yet
- Aging Theories: By: Megah AndrianyDocument28 pagesAging Theories: By: Megah Andrianyborin123No ratings yet
- Biology Investigatory ProjectDocument11 pagesBiology Investigatory Projectshortsprime2No ratings yet
- Mammalian Cell Behavior On Hydrophobic Substrates Influence of Surface PropertiesDocument16 pagesMammalian Cell Behavior On Hydrophobic Substrates Influence of Surface PropertiesManoj Kumar pandreNo ratings yet
- Physics Answer+key 01jpa,+02jpaDocument5 pagesPhysics Answer+key 01jpa,+02jpaHardik TiwariNo ratings yet
- First Strand cDNA Synthesis Protocols (E6300) - NEBDocument1 pageFirst Strand cDNA Synthesis Protocols (E6300) - NEBAldwin AdiongNo ratings yet
- Lab Grown MeatDocument17 pagesLab Grown MeatSaya SufiaNo ratings yet
- Urban Ecology Australia - Do We Fit On The PlanetDocument9 pagesUrban Ecology Australia - Do We Fit On The PlanetRique BenitesNo ratings yet
- Evolution ReviewDocument8 pagesEvolution Reviewn.misovicNo ratings yet