Professional Documents
Culture Documents
Mind Map Organic
Mind Map Organic
Uploaded by
Ismaniza IsmailCopyright:
Available Formats
You might also like
- Organic Chemistry Synthesis IedxcelDocument10 pagesOrganic Chemistry Synthesis IedxcelAliya Rahman100% (2)
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Document1 pageA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- GMP GR: Reaction Chart For AlkanesDocument3 pagesGMP GR: Reaction Chart For AlkanesManoj DesaiNo ratings yet
- Test 1Document9 pagesTest 1Julie Anne CristalesNo ratings yet
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- 12 Organic SynthesisDocument8 pages12 Organic SynthesisDanyal AhmadNo ratings yet
- 6 14 Organic SynthesisDocument8 pages6 14 Organic SynthesisPedro Moreno de SouzaNo ratings yet
- Alkanes Alkenes AlkynesDocument2 pagesAlkanes Alkenes AlkynesGAMEPORIUMNo ratings yet
- Organic Chemistry PosterDocument1 pageOrganic Chemistry Poster텅텅No ratings yet
- Alcohols CIE 9701 As Level Reaction Scheme 1Document1 pageAlcohols CIE 9701 As Level Reaction Scheme 1Daniel MulipolaNo ratings yet
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (6)
- 6 2 5 Revision Guides Organic SynthesisDocument5 pages6 2 5 Revision Guides Organic SynthesisAddan AddanNo ratings yet
- Reagent ListDocument5 pagesReagent ListAditya VermaNo ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Organic ConversionDocument9 pagesOrganic ConversionAnonymous lmpvRsaz90% (1)
- Synthetic Routes PDFDocument1 pageSynthetic Routes PDFjohn spencerNo ratings yet
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnNo ratings yet
- Substitution Reaction, Reflux Potassium /sodium Hydroxide (Koh /naoh)Document1 pageSubstitution Reaction, Reflux Potassium /sodium Hydroxide (Koh /naoh)Abed AymanNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Catalyst Note: (PT, Ni, PD)Document8 pagesCatalyst Note: (PT, Ni, PD)Justin Victor AngNo ratings yet
- MBD Toolkit ADocument1 pageMBD Toolkit AOCRChemistrySaltersNo ratings yet
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- Aliphatic Organic Chemistry Mindmap PDFDocument1 pageAliphatic Organic Chemistry Mindmap PDFIsuru ThenabaduNo ratings yet
- 3.14 Revision Guide Organic Synthesis AqaDocument7 pages3.14 Revision Guide Organic Synthesis AqaRutba SafdarNo ratings yet
- Iit Reductions PDFDocument71 pagesIit Reductions PDFAshish SinghNo ratings yet
- Organic Chemistry Reaction Summary SheetDocument30 pagesOrganic Chemistry Reaction Summary SheetKylo RenNo ratings yet
- 2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Document17 pages2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Anarkin FitriNo ratings yet
- Compound Reagent Product Type of Reaction: SCCH121: Class Activity 1: Organic ReactionsDocument2 pagesCompound Reagent Product Type of Reaction: SCCH121: Class Activity 1: Organic ReactionsNuage DoréNo ratings yet
- Organic RevisionDocument4 pagesOrganic RevisionalicejessicapreesNo ratings yet
- EliminationDocument5 pagesEliminationkhanaazif915No ratings yet
- Alcohols Phenols and EthersDocument1 pageAlcohols Phenols and EthersNitisha GuptaNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFTanishq VermaNo ratings yet
- HaloalkanesDocument1 pageHaloalkanesSahil MenghaniNo ratings yet
- Hydrocarbon: GMP GRDocument30 pagesHydrocarbon: GMP GRVinod AgrawalNo ratings yet
- CHM 2210L Separation of An Acid-Neutral Mixture: The Problem To Be InvestigatedDocument9 pagesCHM 2210L Separation of An Acid-Neutral Mixture: The Problem To Be InvestigateddwiNo ratings yet
- Weapon 4Document1 pageWeapon 4md.muhibmusabbir1101No ratings yet
- Reactions Unit 2 ChemDocument4 pagesReactions Unit 2 Chemsmithsashay74No ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- CHM 203 Carbonyl CompoundsDocument46 pagesCHM 203 Carbonyl Compoundsajibolaakorede20No ratings yet
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Hydrocarbon (12th)Document22 pagesHydrocarbon (12th)Raju SinghNo ratings yet
- 03 Hydro (Alkanes Theory 01)Document16 pages03 Hydro (Alkanes Theory 01)ayushNo ratings yet
- Chapter 10 - Carboxilic Acids 2022Document47 pagesChapter 10 - Carboxilic Acids 2022Hoài Nguyễn Phan VũNo ratings yet
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982100% (1)
- Heterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisDocument30 pagesHeterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisTaciturnoait NihilistaNo ratings yet
- Reduction, Oxidation - Hydrolysis Theory PDFDocument14 pagesReduction, Oxidation - Hydrolysis Theory PDFGOURISH AGRAWALNo ratings yet
- Hydrocarbon 13 THDocument20 pagesHydrocarbon 13 THRaju SinghNo ratings yet
- HYDROCARBONDocument31 pagesHYDROCARBONRaghav VohraNo ratings yet
- Carboxylic & DerivtDocument7 pagesCarboxylic & DerivtNanda NaimahNo ratings yet
- 2018 l3 Organic Reaction Scheme Version 2Document1 page2018 l3 Organic Reaction Scheme Version 2NUR SYAFIQAH BINTI MD REJABNo ratings yet
- Greg Marshall Catalytic Reforming For Aromatic Production PDFDocument19 pagesGreg Marshall Catalytic Reforming For Aromatic Production PDFArash AbbasiNo ratings yet
- Hydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Document22 pagesHydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Prince DigvijayNo ratings yet
- Chem-GA 1311 Spring 2021 F, ReductionDocument38 pagesChem-GA 1311 Spring 2021 F, ReductionHY-11 Đỗ Quốc TiệpNo ratings yet
- Handbook of Palladium-Catalysed Organic ReactionsFrom EverandHandbook of Palladium-Catalysed Organic ReactionsJ. C. FiaudNo ratings yet
- HydrocarbonsDocument80 pagesHydrocarbonsina stanevaNo ratings yet
- Alkyl HalidesDocument75 pagesAlkyl HalidesVikas GargNo ratings yet
- Previous Hse Questions and Answers of The Chapter "Hydrocarbons"Document10 pagesPrevious Hse Questions and Answers of The Chapter "Hydrocarbons"Muhammed SadiqNo ratings yet
- Common Model Exam Set-IX (2078-7-6) SolDocument12 pagesCommon Model Exam Set-IX (2078-7-6) SolPrepladder ChyNo ratings yet
- Chemistry: Pearson Edexcel International GCSE (9-1)Document5 pagesChemistry: Pearson Edexcel International GCSE (9-1)Syed Moinul HoqueNo ratings yet
- Thermal Cracking of N-Butylcyclohexane at High Pressure (100 Bar) - Part 1: Experimental StudyDocument16 pagesThermal Cracking of N-Butylcyclohexane at High Pressure (100 Bar) - Part 1: Experimental StudyDarwin ANo ratings yet
- Organics QuestionsDocument3 pagesOrganics Questionsnairamathrawala3000No ratings yet
- Grade 11 Chemistry Lesson 2Document4 pagesGrade 11 Chemistry Lesson 2Rokeish RoweNo ratings yet
- Alkyl Halides: S5 Chemistry 29/NOV/2021Document31 pagesAlkyl Halides: S5 Chemistry 29/NOV/2021Nelima Stella mercyNo ratings yet
- Mocks 11C P2 2021Document16 pagesMocks 11C P2 2021adilNo ratings yet
- Chemistry FileDocument41 pagesChemistry FilePreetiNo ratings yet
- Mind Map ChemistryDocument3 pagesMind Map ChemistryTheesha SophieNo ratings yet
- Physical Science Scope 2021 Grade 12Document3 pagesPhysical Science Scope 2021 Grade 12Bandile MhlongoNo ratings yet
- CBSE Sample Question Papers For Class 12 Chemistry 2020Document16 pagesCBSE Sample Question Papers For Class 12 Chemistry 2020Emtiaz AnsariNo ratings yet
- Practice PacketDocument20 pagesPractice PacketTheresa Rebullos BileNo ratings yet
- Combustion of FuelsDocument6 pagesCombustion of FuelsRobby Royce Casida100% (1)
- Chapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionDocument37 pagesChapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionSCReddyNo ratings yet
- 5 6294291619013198032Document499 pages5 6294291619013198032juma indah pertiwiNo ratings yet
- DKHDA ReactionDocument14 pagesDKHDA ReactionBhavesh PansuriyaNo ratings yet
- Organic Chemistry: Alkene NotesDocument11 pagesOrganic Chemistry: Alkene NotesDommie FranklinNo ratings yet
- Organic Chemistry Alkenes WorksheetDocument2 pagesOrganic Chemistry Alkenes Worksheetoc100% (1)
- Chapter 2 AlkanesDocument6 pagesChapter 2 AlkanesShan TiNo ratings yet
- Homework Booklet (4, S)Document55 pagesHomework Booklet (4, S)VarshLokNo ratings yet
- Physical Properties and Chemical Reactions ofDocument9 pagesPhysical Properties and Chemical Reactions ofTUANA DURMAYÜKSELNo ratings yet
- (Teacher Version) Final Revision Aqa Chemistry International Gcse 2Document17 pages(Teacher Version) Final Revision Aqa Chemistry International Gcse 2montada mansorNo ratings yet
- Science 2015 PDFDocument15 pagesScience 2015 PDFashwaniNo ratings yet
- Pharmaceutical Chemistry Chapter 4 Organic Chemisty NotesDocument10 pagesPharmaceutical Chemistry Chapter 4 Organic Chemisty Notesbf327626No ratings yet
- Acrylonitrile: Guidelines For The Distribution ofDocument79 pagesAcrylonitrile: Guidelines For The Distribution ofJose LopezNo ratings yet
Mind Map Organic
Mind Map Organic
Uploaded by
Ismaniza IsmailOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mind Map Organic
Mind Map Organic
Uploaded by
Ismaniza IsmailCopyright:
Available Formats
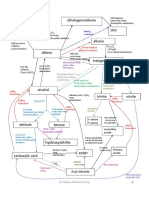
CATALYTIC HYDROGENATION Ni HEAT CCl4
HALOGENATION DIHALOALKANE
ALKANE ALKENE
HYDROHALOGENATION DEHYDRATION H2O HALOHYDRIN
HYDRATION
FREE RADICAL ELECTROPHILIC ELIMINATION RXN
SUSBSTITUTION ADDITION RXN
ELECTROPHILIC
CONC H2SO4
MARKONIKOV’S RULE ELIMINATION RXN ADDITION RXN
UV light
SAYTZEFF’S RULE KETONE
DEHYDROHALOGANATION H3PO4, HEAT
Cl2 / Br2 KMnO4 /H+
SAYTZEFF’S RULE MARKONIKOV’S RULE
OXIDATION RXN
NaBH4
REDUCTION RXN
NUCLEOPHILIC HALOGEN HALIDE INORGANIC HALIDES
HALO
SUBSTITUTION @ HCl @ PCl5 2o
ALKANE
PCC in DCM
ALCOHOL CYANO
NUCLEOPHILIC NaOH OXIDATION RXN + HCN
REFLUX 1o HYDRIN
SUBSTITUTION (in aqueous)
KMnO4/H+
+
Mg REDUCTION RXN
CH2O
DRY ETHER NaBH4
DRY ETHER
NUCLEOPHILIC H3O+ REDUCTION RXN LiAlH4 H3O+ ALDEHYDE
SUBSTITUTION GRIGNARD CARBOXYLIC
REAGENTS ACID
KCN (in ethanol)
OXIDATION RXN KMnO4/H+
CO2 H3O+
DRY ETHER
CONC H2SO4
NITRILE SOCl2
DRY ETHER NH3
ESTER ACYL
CHLORIDE AMIDE
H3O+
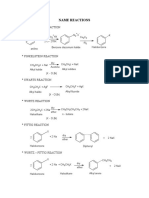
TESTING OF ALCOHOL
1. Lucas test
- 30 alcohol – cloudy immediately
- 20 alcohol – cloudy in 5 min
- 10 alcohol – no cloudiness
TESTING OF ALKENE
2. Oxidation KMnO4
1. Br2 in inert solvent or dilute bromine water - 10 & 20 alcohol – Purple to colorless
- Brown to colorless - 30 alcohol – no change
- alkene (in inert solvent) → dihaloalkane
- Alkene (Br2 water) → halohydrin 3. Iodoform
- Positive results for methyl alcohol & ethanol
2. Cold, dilute, alkaline KMnO4 (Baeyer’s test) - Pale yellow solid
- Purple to brown ppt
- Alkene → diol
TESTING OF CARBONYL
3. Hot, acidified KMnO4
- Purple to colorless 1. Brady’s reagent
10 C – CO2 - 2,4-dinitropehylhydrazine (2,4-DNP)
20 C – COOH - Turns to yellow-orange ppt
30 C – KETONE
2. Tollen’s reagent (silver mirror test)
- Positive results against aldehyde → silver mirror
3. Fehling’s reagent
- Positive result against aldehyde
- Deep blue → brick red
4. Iodoform
- Positive results for ethanal & methyl ketone
- Pale yellow solid
You might also like
- Organic Chemistry Synthesis IedxcelDocument10 pagesOrganic Chemistry Synthesis IedxcelAliya Rahman100% (2)
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Document1 pageA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- GMP GR: Reaction Chart For AlkanesDocument3 pagesGMP GR: Reaction Chart For AlkanesManoj DesaiNo ratings yet
- Test 1Document9 pagesTest 1Julie Anne CristalesNo ratings yet
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- 12 Organic SynthesisDocument8 pages12 Organic SynthesisDanyal AhmadNo ratings yet
- 6 14 Organic SynthesisDocument8 pages6 14 Organic SynthesisPedro Moreno de SouzaNo ratings yet
- Alkanes Alkenes AlkynesDocument2 pagesAlkanes Alkenes AlkynesGAMEPORIUMNo ratings yet
- Organic Chemistry PosterDocument1 pageOrganic Chemistry Poster텅텅No ratings yet
- Alcohols CIE 9701 As Level Reaction Scheme 1Document1 pageAlcohols CIE 9701 As Level Reaction Scheme 1Daniel MulipolaNo ratings yet
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (6)
- 6 2 5 Revision Guides Organic SynthesisDocument5 pages6 2 5 Revision Guides Organic SynthesisAddan AddanNo ratings yet
- Reagent ListDocument5 pagesReagent ListAditya VermaNo ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Organic ConversionDocument9 pagesOrganic ConversionAnonymous lmpvRsaz90% (1)
- Synthetic Routes PDFDocument1 pageSynthetic Routes PDFjohn spencerNo ratings yet
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnNo ratings yet
- Substitution Reaction, Reflux Potassium /sodium Hydroxide (Koh /naoh)Document1 pageSubstitution Reaction, Reflux Potassium /sodium Hydroxide (Koh /naoh)Abed AymanNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Catalyst Note: (PT, Ni, PD)Document8 pagesCatalyst Note: (PT, Ni, PD)Justin Victor AngNo ratings yet
- MBD Toolkit ADocument1 pageMBD Toolkit AOCRChemistrySaltersNo ratings yet
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- Aliphatic Organic Chemistry Mindmap PDFDocument1 pageAliphatic Organic Chemistry Mindmap PDFIsuru ThenabaduNo ratings yet
- 3.14 Revision Guide Organic Synthesis AqaDocument7 pages3.14 Revision Guide Organic Synthesis AqaRutba SafdarNo ratings yet
- Iit Reductions PDFDocument71 pagesIit Reductions PDFAshish SinghNo ratings yet
- Organic Chemistry Reaction Summary SheetDocument30 pagesOrganic Chemistry Reaction Summary SheetKylo RenNo ratings yet
- 2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Document17 pages2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Anarkin FitriNo ratings yet
- Compound Reagent Product Type of Reaction: SCCH121: Class Activity 1: Organic ReactionsDocument2 pagesCompound Reagent Product Type of Reaction: SCCH121: Class Activity 1: Organic ReactionsNuage DoréNo ratings yet
- Organic RevisionDocument4 pagesOrganic RevisionalicejessicapreesNo ratings yet
- EliminationDocument5 pagesEliminationkhanaazif915No ratings yet
- Alcohols Phenols and EthersDocument1 pageAlcohols Phenols and EthersNitisha GuptaNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFTanishq VermaNo ratings yet
- HaloalkanesDocument1 pageHaloalkanesSahil MenghaniNo ratings yet
- Hydrocarbon: GMP GRDocument30 pagesHydrocarbon: GMP GRVinod AgrawalNo ratings yet
- CHM 2210L Separation of An Acid-Neutral Mixture: The Problem To Be InvestigatedDocument9 pagesCHM 2210L Separation of An Acid-Neutral Mixture: The Problem To Be InvestigateddwiNo ratings yet
- Weapon 4Document1 pageWeapon 4md.muhibmusabbir1101No ratings yet
- Reactions Unit 2 ChemDocument4 pagesReactions Unit 2 Chemsmithsashay74No ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- CHM 203 Carbonyl CompoundsDocument46 pagesCHM 203 Carbonyl Compoundsajibolaakorede20No ratings yet
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Hydrocarbon (12th)Document22 pagesHydrocarbon (12th)Raju SinghNo ratings yet
- 03 Hydro (Alkanes Theory 01)Document16 pages03 Hydro (Alkanes Theory 01)ayushNo ratings yet
- Chapter 10 - Carboxilic Acids 2022Document47 pagesChapter 10 - Carboxilic Acids 2022Hoài Nguyễn Phan VũNo ratings yet
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982100% (1)
- Heterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisDocument30 pagesHeterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisTaciturnoait NihilistaNo ratings yet
- Reduction, Oxidation - Hydrolysis Theory PDFDocument14 pagesReduction, Oxidation - Hydrolysis Theory PDFGOURISH AGRAWALNo ratings yet
- Hydrocarbon 13 THDocument20 pagesHydrocarbon 13 THRaju SinghNo ratings yet
- HYDROCARBONDocument31 pagesHYDROCARBONRaghav VohraNo ratings yet
- Carboxylic & DerivtDocument7 pagesCarboxylic & DerivtNanda NaimahNo ratings yet
- 2018 l3 Organic Reaction Scheme Version 2Document1 page2018 l3 Organic Reaction Scheme Version 2NUR SYAFIQAH BINTI MD REJABNo ratings yet
- Greg Marshall Catalytic Reforming For Aromatic Production PDFDocument19 pagesGreg Marshall Catalytic Reforming For Aromatic Production PDFArash AbbasiNo ratings yet
- Hydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Document22 pagesHydrocarbon: Ashwani Tyagi Sir (Code: ATJEE)Prince DigvijayNo ratings yet
- Chem-GA 1311 Spring 2021 F, ReductionDocument38 pagesChem-GA 1311 Spring 2021 F, ReductionHY-11 Đỗ Quốc TiệpNo ratings yet
- Handbook of Palladium-Catalysed Organic ReactionsFrom EverandHandbook of Palladium-Catalysed Organic ReactionsJ. C. FiaudNo ratings yet
- HydrocarbonsDocument80 pagesHydrocarbonsina stanevaNo ratings yet
- Alkyl HalidesDocument75 pagesAlkyl HalidesVikas GargNo ratings yet
- Previous Hse Questions and Answers of The Chapter "Hydrocarbons"Document10 pagesPrevious Hse Questions and Answers of The Chapter "Hydrocarbons"Muhammed SadiqNo ratings yet
- Common Model Exam Set-IX (2078-7-6) SolDocument12 pagesCommon Model Exam Set-IX (2078-7-6) SolPrepladder ChyNo ratings yet
- Chemistry: Pearson Edexcel International GCSE (9-1)Document5 pagesChemistry: Pearson Edexcel International GCSE (9-1)Syed Moinul HoqueNo ratings yet
- Thermal Cracking of N-Butylcyclohexane at High Pressure (100 Bar) - Part 1: Experimental StudyDocument16 pagesThermal Cracking of N-Butylcyclohexane at High Pressure (100 Bar) - Part 1: Experimental StudyDarwin ANo ratings yet
- Organics QuestionsDocument3 pagesOrganics Questionsnairamathrawala3000No ratings yet
- Grade 11 Chemistry Lesson 2Document4 pagesGrade 11 Chemistry Lesson 2Rokeish RoweNo ratings yet
- Alkyl Halides: S5 Chemistry 29/NOV/2021Document31 pagesAlkyl Halides: S5 Chemistry 29/NOV/2021Nelima Stella mercyNo ratings yet
- Mocks 11C P2 2021Document16 pagesMocks 11C P2 2021adilNo ratings yet
- Chemistry FileDocument41 pagesChemistry FilePreetiNo ratings yet
- Mind Map ChemistryDocument3 pagesMind Map ChemistryTheesha SophieNo ratings yet
- Physical Science Scope 2021 Grade 12Document3 pagesPhysical Science Scope 2021 Grade 12Bandile MhlongoNo ratings yet
- CBSE Sample Question Papers For Class 12 Chemistry 2020Document16 pagesCBSE Sample Question Papers For Class 12 Chemistry 2020Emtiaz AnsariNo ratings yet
- Practice PacketDocument20 pagesPractice PacketTheresa Rebullos BileNo ratings yet
- Combustion of FuelsDocument6 pagesCombustion of FuelsRobby Royce Casida100% (1)
- Chapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionDocument37 pagesChapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionSCReddyNo ratings yet
- 5 6294291619013198032Document499 pages5 6294291619013198032juma indah pertiwiNo ratings yet
- DKHDA ReactionDocument14 pagesDKHDA ReactionBhavesh PansuriyaNo ratings yet
- Organic Chemistry: Alkene NotesDocument11 pagesOrganic Chemistry: Alkene NotesDommie FranklinNo ratings yet
- Organic Chemistry Alkenes WorksheetDocument2 pagesOrganic Chemistry Alkenes Worksheetoc100% (1)
- Chapter 2 AlkanesDocument6 pagesChapter 2 AlkanesShan TiNo ratings yet
- Homework Booklet (4, S)Document55 pagesHomework Booklet (4, S)VarshLokNo ratings yet
- Physical Properties and Chemical Reactions ofDocument9 pagesPhysical Properties and Chemical Reactions ofTUANA DURMAYÜKSELNo ratings yet
- (Teacher Version) Final Revision Aqa Chemistry International Gcse 2Document17 pages(Teacher Version) Final Revision Aqa Chemistry International Gcse 2montada mansorNo ratings yet
- Science 2015 PDFDocument15 pagesScience 2015 PDFashwaniNo ratings yet
- Pharmaceutical Chemistry Chapter 4 Organic Chemisty NotesDocument10 pagesPharmaceutical Chemistry Chapter 4 Organic Chemisty Notesbf327626No ratings yet
- Acrylonitrile: Guidelines For The Distribution ofDocument79 pagesAcrylonitrile: Guidelines For The Distribution ofJose LopezNo ratings yet