Professional Documents
Culture Documents
Formulation Activity OxidesHydrides Solutions
Formulation Activity OxidesHydrides Solutions
Uploaded by
Alfredo Sanz0 ratings0% found this document useful (0 votes)
2 views2 pagesOriginal Title
Formulation Activity OxidesHydrides Solutions (7)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
2 views2 pagesFormulation Activity OxidesHydrides Solutions
Formulation Activity OxidesHydrides Solutions
Uploaded by
Alfredo SanzCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
IES Virgen del Espino Activities.
2nd ESO
Formulation and Nomenclature
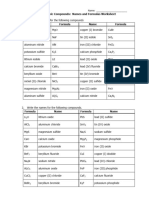
1.- Write the name (both nomenclatures in each compound) of the following compounds:
Formula Sistematic Stock Traditional
a) FeO Iron monoxide Iron (II) oxide ______________________
b) N2 O5 Dinitrogen pentoxide Niktrogen (V) oxide _______________________
c) CO2 Carbon dioxide Carbon (IV) oxide ____________________
d) CaH2 Calcium dihydride Calcium hydride _____________________
e) MgO Magnesium monoxide Magnesium oxide ______________________
f) SnO2 Tin dioxide Tin (IV) oxide _____________________
g) SiH4 Silicon tetrahydride Silane
h) PH3 Phosphorous Trihydride ______________________ Phosphane
i) I2O3 Diiodine trioxide Iodine (III) oxide _________________
j) SO2 Sulphur dioxide Sulphur (IV) oxide ___________________
k) Br2O7 Dibromine heptoxide Bromine (VII) oxide _____________________
l) Fe2O3 Diiron trioxide Iron (III) oxide ______________________
m) NaH Sodium monohydride Sodium Hydride _____________________
n) ZnO Zinc monoxide Zinc Oxide __________________________
o) SO3 Sulphur trioxide Sulphur (VI) oxide
p) B2O3 Diboron trioxide Boron oxide
q) FeH3 Iron trihydride Iron (III) hydride
r) P2O3 Diphosphorous trioxide Phosphorous (III) oxide
s) NH3 Nitrogen trihydride Ammonia
t) Cl2O Dichlorine monoxide Chlorine (I) oxide
u) K2O Dipotassium monoxide Potassium oxide
2.- Write the formula of the following compounds:
a) Diiron trioxide………….Fe2O3
b) Ammonia………………..NH3
c) Sulphur trioxide………SO3
d) Caesium hydride……..CsH
e) Nickel (II) oxide……….NiO
f) Manganese (IV) hydride………MnH4
g) Dibromine pentaoxide……..Br2O5
h) Phosphorous (V) oxide…..P2O5
i) Chromium (VI) oxide…….CrO3
j) Manganese (III) hydride…..MnH3
k) Aluminium oxide……Al2O3
l) Carbon monoxide…CO
m) Tin (IV) oxide……………SnO2
n) Potassium hydride……..KH
o) Cobalt (III) hydride…….CoH3
p) Dinickel trioxide Ni2O3
q) Cadmium (II) hydride CdH2
r) Sulphur (VI) oxide SO3
s) Lithium oxide Li2O
t) Chromium trioxide CrO3
u) Chromium (VI) oxide CrO3
You might also like
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- NES M0301-2009 Substance Use RestrictionsDocument118 pagesNES M0301-2009 Substance Use RestrictionsMaria P.75% (4)
- En 14214 BiodieselDocument2 pagesEn 14214 BiodieselsmsNo ratings yet
- Bio Chem Labbb EditDocument37 pagesBio Chem Labbb EditAlbert Azura100% (1)
- Ionic Compound Formula Writing-2Document5 pagesIonic Compound Formula Writing-2lalNo ratings yet
- Naming Ionic Compounds V DD CHDocument2 pagesNaming Ionic Compounds V DD CHVince Carl BalbuenaNo ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- ATP Star 2Document28 pagesATP Star 2Gowri ShankarNo ratings yet
- Disinfection For PH 2Document73 pagesDisinfection For PH 2Boas WayneNo ratings yet
- w308 Naming Ionic Compounds Worksheet 2Document2 pagesw308 Naming Ionic Compounds Worksheet 2Trust Kachingwe KachingweNo ratings yet
- Naming Compounds: Name: Nina Mariz D. Pacilan Grade & Section: 9-AirDocument4 pagesNaming Compounds: Name: Nina Mariz D. Pacilan Grade & Section: 9-AirNiña Mariz PacilanNo ratings yet
- Writing Ionic Compound Formulas IV: Licensed Via Creative Commons License A Cooperative Effort Between The andDocument2 pagesWriting Ionic Compound Formulas IV: Licensed Via Creative Commons License A Cooperative Effort Between The andmikeNo ratings yet
- Even More Naming Ionic Compounds: This Work Is Licensed Under A and IsDocument2 pagesEven More Naming Ionic Compounds: This Work Is Licensed Under A and IsNeeta PandeyNo ratings yet
- Chemical Formula Writing Worksheet2Document2 pagesChemical Formula Writing Worksheet2عابدهعلي100% (1)
- Naming Ionic Compounds III DD Ch1Document2 pagesNaming Ionic Compounds III DD Ch1anasabohloNo ratings yet
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- Ionic Compound Formula Writing WorksheetDocument6 pagesIonic Compound Formula Writing WorksheetIngrid ElizabethNo ratings yet
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Formulas and Names of Ionic CompoundsDocument3 pagesFormulas and Names of Ionic CompoundsMandanas GabrielNo ratings yet
- Annotated Annotated Kami%20Export%20 %20ionic%20compound%20formula%20writing 1%20%283%29%20%281%29Document1 pageAnnotated Annotated Kami%20Export%20 %20ionic%20compound%20formula%20writing 1%20%283%29%20%281%29taniahmtylerNo ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- More Nomenclature PracticeDocument2 pagesMore Nomenclature PracticeeapicciottoNo ratings yet
- Nomenclature Flashcard ActivityDocument11 pagesNomenclature Flashcard ActivityNancy AndrawesNo ratings yet
- Worksheet Ionic Compound Formula WritingDocument5 pagesWorksheet Ionic Compound Formula Writingyarielaale22No ratings yet
- Test 1 Formula of IonsDocument6 pagesTest 1 Formula of IonsSEAW FUI MINGNo ratings yet
- ACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGDocument4 pagesACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGdavid burneNo ratings yet
- ACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGDocument4 pagesACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGdavid burneNo ratings yet
- ACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGDocument4 pagesACFrOgBkYjdU94Prdnt5lAjyEYwo7KUcrruvAO-FhZS14M42VCT8Mm0G5ldoJSx4Wt0L2d00HlH6B SBh8NGUlfvsxxCacc FqVk44GWsvba5vC7Gt i4BMvAK5m81mF2BztlA1yc9sY66iBV1sGdavid burneNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- CLASS IX (2021-2022) Chemistry Revisionsheet (Po1-Part - 2)Document2 pagesCLASS IX (2021-2022) Chemistry Revisionsheet (Po1-Part - 2)priya srivastavaNo ratings yet
- Microsoft Word - Ionic-CovalentNameRace1Document2 pagesMicrosoft Word - Ionic-CovalentNameRace1cen BsitNo ratings yet
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedJaiy HingcoNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedMca ImusNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights Reservedpao manaligodNo ratings yet
- Naming Covalent CompoundsDocument2 pagesNaming Covalent CompoundsCrisJoy DiuyanNo ratings yet
- Mixed Ionic and Covalent Naming III: Name The Following Chemical CompoundsDocument2 pagesMixed Ionic and Covalent Naming III: Name The Following Chemical CompoundsAndrea C.No ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedManohar GarimellaNo ratings yet
- C3 Exercise 1Document8 pagesC3 Exercise 1Noor Liyana Ahmad FuadNo ratings yet
- Naming Chemical CompoundsDocument6 pagesNaming Chemical CompoundsClara BangunNo ratings yet
- G10 Science: Class 1 HomeworkDocument4 pagesG10 Science: Class 1 HomeworkEthan LiuNo ratings yet
- Jordan Paddock - Writing Multivalent Names and Formulas WorksheetDocument1 pageJordan Paddock - Writing Multivalent Names and Formulas Worksheetapi-675312232No ratings yet
- Compound Name Type of Bond Chemical Formula Alternate Name (If Applicable)Document2 pagesCompound Name Type of Bond Chemical Formula Alternate Name (If Applicable)anon-579447No ratings yet
- 1.6A Molecular Compounds, Extra ExercisesDocument1 page1.6A Molecular Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Naming Inorganic Compound Practice SheetDocument4 pagesNaming Inorganic Compound Practice SheetWichel AnnNo ratings yet
- SaltsDocument2 pagesSaltsignacymarcinkiewicz1No ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- Task #6 - Naming Chemical Formulas and Balancing EquationDocument2 pagesTask #6 - Naming Chemical Formulas and Balancing EquationPAUL AYRUM SALESNo ratings yet
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- w306 Naming Ionic Compounds WorksheetDocument2 pagesw306 Naming Ionic Compounds Worksheetzafarchem_iqbal100% (2)
- Nomenclature WorksheetDocument3 pagesNomenclature WorksheetKlorin Min100% (1)
- Naming Compounds S2 6Document3 pagesNaming Compounds S2 6mehtadhruv1325No ratings yet
- Naming Chemical Compounds WorksheetDocument3 pagesNaming Chemical Compounds Worksheetjeetha margaretNo ratings yet
- Compressed Sheet With Carbon Fibers, NBR Binder: ApplicationDocument2 pagesCompressed Sheet With Carbon Fibers, NBR Binder: ApplicationEmmanuelNo ratings yet
- 5.attachment With KVKDocument15 pages5.attachment With KVKSamuel DavisNo ratings yet
- Formulating Anhydride-Cured Epoxy Systems: Technical BulletinDocument9 pagesFormulating Anhydride-Cured Epoxy Systems: Technical BulletinmelsabaeNo ratings yet
- May 4, 2012 JNTUH, Hyderabad 1Document72 pagesMay 4, 2012 JNTUH, Hyderabad 1Gandla Ravi ThejaNo ratings yet
- Price List Spectra - Update 8 Juni 2023Document11 pagesPrice List Spectra - Update 8 Juni 2023Eko PrasetyoNo ratings yet
- Process For Surface Sizing PaperDocument7 pagesProcess For Surface Sizing PaperRakeshNo ratings yet
- Synthesis of Zeolite A A ReviewDocument6 pagesSynthesis of Zeolite A A ReviewSohel SurtiNo ratings yet
- Ibs To Ich PR Ctice ProblemsDocument4 pagesIbs To Ich PR Ctice ProblemsgyeonggNo ratings yet
- SOP - Quenching Pyrophoric MaterialsDocument5 pagesSOP - Quenching Pyrophoric Materialsdhavalesh1No ratings yet
- Organic Crop ProductionDocument3 pagesOrganic Crop ProductionDimple EstacioNo ratings yet
- 2Document6 pages2Kuo Garol Sarong100% (1)
- Biodegradability of PVAcDocument36 pagesBiodegradability of PVAcThoại Nguyễn QuốcNo ratings yet
- CAPE PhotosynthesisDocument20 pagesCAPE PhotosynthesisIsheba Warren100% (24)
- Chapter 6 - Biological Method - 2 FinalDocument96 pagesChapter 6 - Biological Method - 2 FinalDang Nguyen Anh HuyNo ratings yet
- Answers To Study QuestionsDocument2 pagesAnswers To Study Questionsaarmeor100% (3)
- Ureas and Thioureas As Asymmetric OrganocatalystsDocument60 pagesUreas and Thioureas As Asymmetric OrganocatalystsAlex FNo ratings yet
- An Improved Isolation of Trimyristin From Myristica Fragrans As A Renewable Feedstock With The Assistance of Novel Cationic Gemini SurfactantDocument16 pagesAn Improved Isolation of Trimyristin From Myristica Fragrans As A Renewable Feedstock With The Assistance of Novel Cationic Gemini SurfactantNabila PutriNo ratings yet
- Content of Unsaturated Fats Measured by The Iodine Number in Coconut and Sunflower Oil After BeingDocument13 pagesContent of Unsaturated Fats Measured by The Iodine Number in Coconut and Sunflower Oil After BeingMM DomìN8RNo ratings yet
- Organic Reaction Mechanisms - A Step by Step Approach, Second EditionDocument517 pagesOrganic Reaction Mechanisms - A Step by Step Approach, Second Editionmehrdad63100% (8)
- Karmarkar Effectsandculture 1969Document9 pagesKarmarkar Effectsandculture 1969Trechabel OlaniganNo ratings yet
- Ineos Chequered Environmental Track Record in EuropeDocument27 pagesIneos Chequered Environmental Track Record in EuropeFood and Water WatchNo ratings yet
- NEET - Halo Alkanes and Halo Arenes Practice PaperDocument3 pagesNEET - Halo Alkanes and Halo Arenes Practice PaperGanga DharaNo ratings yet
- Amrita LabDocument6 pagesAmrita LabSweta SumanNo ratings yet
- Compatibility Characterization of Poly (Lactic Acid) / Poly (Propylene Carbonate) BlendsDocument8 pagesCompatibility Characterization of Poly (Lactic Acid) / Poly (Propylene Carbonate) BlendsMuhammad Mushtaq AliNo ratings yet
- Carbon Compound Flow ChartDocument4 pagesCarbon Compound Flow ChartpkrajenpillaygmailcomNo ratings yet