Professional Documents
Culture Documents
Molecular Geometry Chart (VSEPR Shapes)
Molecular Geometry Chart (VSEPR Shapes)
Uploaded by
laila SheashaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Molecular Geometry Chart (VSEPR Shapes)
Molecular Geometry Chart (VSEPR Shapes)
Uploaded by
laila SheashaCopyright:
Available Formats
© Adrian Dingle’s Chemistry Pages 2004, 2005, 2006. All rights reserved.
These materials may NOT be copied or redistributed in any way, except for individual class instruction.
Revised August 2006
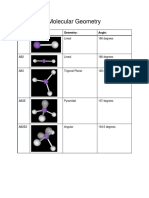
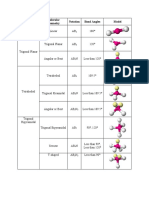
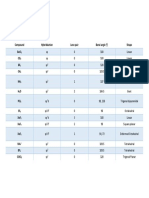
Number of electron
pairs around central Full description of the molecule
atom
Geometry of Geometry of
BONDING (B) LONE (E) Example Bond angles o 3D Shape Type
Electron Pairs Atoms
2 0 BeCl2 180 Linear Linear AB2
Trigonal Trigonal
3 0 BF3 120 AB3
planar Planar
Slightly less Trigonal Bent or
2 1 SO2 AB2E
than 120 planar V Shaped
4 0 CH4 109.5 Tetrahedral Tetrahedral AB4
Trigonal
3 1 NH3 107.5 Tetrahedral AB3E
Pyramidal
Bent or
2 2 H2O 104.5 Tetrahedral AB2E2
V Shaped
120 in plane.
90 Trigonal Trigonal
5 0 PCl5 AB5
perpendicular bipyramidal Bipyramidal
to plane
Trigonal
4 1 SF4 Complex Seesaw AB4E
bipyramid
Trigonal
3 2 ClF3 Approx. 90 T-Shaped AB3E2
bipyramidal
Trigonal
2 3 XeF2 180 Linear AB2E3
bipyramid
6 0 SF6 90 Octahedral Octahedral AB6
Square
5 1 BrF5 Approx. 90 Octahedral AB5E
Pyramidal
Square
4 2 XeF4 90 Octahedral AB4E2
Planar
C:\adriandingleschemistrypages.com\apnotes08.doc Page 10 of 21
You might also like
- Vsepr Theory Summary ChartDocument2 pagesVsepr Theory Summary ChartLittle One100% (2)
- Lewis Structures and Molecular GeometryDocument2 pagesLewis Structures and Molecular GeometryrsleoNo ratings yet
- Chemistry WorksheetDocument5 pagesChemistry WorksheetGiezel MadurarNo ratings yet
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjNo ratings yet
- Nota VSEPR PDFDocument1 pageNota VSEPR PDFMarlene GazconNo ratings yet
- Finals L2 Chemistry Part 2 VSEPR TableDocument3 pagesFinals L2 Chemistry Part 2 VSEPR TableRhoda Mae CubillaNo ratings yet
- 09 - Molecular Structure and Covalent Bonding TheoriesDocument10 pages09 - Molecular Structure and Covalent Bonding TheoriesReiVanNo ratings yet
- Vsepr HandoutDocument2 pagesVsepr HandoutAdrianne Jericho ValdezNo ratings yet
- VSEPR Handout PDFDocument2 pagesVSEPR Handout PDFLittle One0% (1)
- Vsepr HandoutDocument2 pagesVsepr Handout20718 LAY BUFFON FERNANDO GROSSONo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryAryanna CamachoNo ratings yet
- VSEPRDocument1 pageVSEPRMuhammad AsifNo ratings yet
- Molecular GeometryDocument4 pagesMolecular Geometryapi-449127308No ratings yet
- Vsepr TheoryDocument6 pagesVsepr Theorydjjagu908No ratings yet
- Shapes of Covalent MoleculesDocument5 pagesShapes of Covalent MoleculesSiya ChiniahNo ratings yet
- 3 AB Trigonal Planar Trigonal Planar 120 Between All BondsDocument5 pages3 AB Trigonal Planar Trigonal Planar 120 Between All BondsVedantNo ratings yet
- Molecular Shapes of Molecular and IonsDocument3 pagesMolecular Shapes of Molecular and IonsP YNo ratings yet
- Electron Domains and Molecular Geometry IBDP ChemistryDocument1 pageElectron Domains and Molecular Geometry IBDP Chemistryyasmeen alkhaterNo ratings yet
- Introduction To MolecularDocument1 pageIntroduction To Molecularclairole quilantangNo ratings yet
- C MOLECULAR SHAPES and Shapes of Organic MoleculesDocument6 pagesC MOLECULAR SHAPES and Shapes of Organic Moleculesshan mackNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- 2..chemical Bonding Theory-12-12Document1 page2..chemical Bonding Theory-12-12Ashish SharmaNo ratings yet
- 360 04 Mol IonsDocument1 page360 04 Mol IonsJonathan Jon ChiaNo ratings yet
- VSEPR TheoryDocument1 pageVSEPR TheorySania SamiNo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica Mamauag0% (1)
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica MamauagNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- Worksheet Vsepr Theory Using The Knowledge of VSEPR Theory, Complete The Table Given BelowDocument2 pagesWorksheet Vsepr Theory Using The Knowledge of VSEPR Theory, Complete The Table Given Belownidhi1478No ratings yet
- Teri C No. Basic Geometry 0 Lone Pair 1 Lone Pair 2 Lone Pairs 3 Lone PairsDocument5 pagesTeri C No. Basic Geometry 0 Lone Pair 1 Lone Pair 2 Lone Pairs 3 Lone PairsShamsiNo ratings yet
- Compound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclDocument1 pageCompound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclSakib KhanNo ratings yet
- Lecture Statics of Rigid BodiesDocument5 pagesLecture Statics of Rigid BodiesalainxanoricoNo ratings yet
- Geometría Molecular PDFDocument1 pageGeometría Molecular PDFGeanellaNo ratings yet
- Chemical Bonding (F Only)Document28 pagesChemical Bonding (F Only)Raju SinghNo ratings yet
- VSEPR and Molecular Geometries (Summery)Document2 pagesVSEPR and Molecular Geometries (Summery)MihadNo ratings yet
- Tips & Tricks Inorganic Chemistry FlashCardsDocument25 pagesTips & Tricks Inorganic Chemistry FlashCardsseemagoyal0206No ratings yet
- PhET VSEPR Simulation (Honors)Document4 pagesPhET VSEPR Simulation (Honors)merryscotNo ratings yet
- Activity Lab 5 ChemistryDocument3 pagesActivity Lab 5 ChemistryElmer EmpeñoNo ratings yet
- 3.10 Molecular CompoundsDocument3 pages3.10 Molecular CompoundsIBRAHIM ABOU EL NAAJNo ratings yet
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- Bond Angle ChartDocument1 pageBond Angle ChartxwenhanNo ratings yet
- Vsepr ChartDocument2 pagesVsepr Chartapi-239855791No ratings yet
- 4.3-VSEPR - Shapes of MoleculesDocument1 page4.3-VSEPR - Shapes of MoleculesStephan MinhNo ratings yet
- Shapes of MoleculesDocument3 pagesShapes of MoleculesAaron Abdur RahimNo ratings yet
- Geometry SheetDocument1 pageGeometry Sheetapi-3697114100% (1)
- PROBLEM 2.124: SolutionDocument14 pagesPROBLEM 2.124: SolutionCharbel Abou KhalilNo ratings yet
- Chemical BondingDocument28 pagesChemical BondingPrince DigvijayNo ratings yet
- Molecular ModelDocument1 pageMolecular ModelPedro SuyuNo ratings yet
- Che2060 Vsepr Geometry Ws KeyDocument5 pagesChe2060 Vsepr Geometry Ws Keyqvcws4h5spNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- VSEPRDocument1 pageVSEPRĐan KhanhNo ratings yet
- Bonding Iedxcel 2Document1 pageBonding Iedxcel 2Kenzy ShahinNo ratings yet
- 230KV Line Feeder at 230/ 110KV Savasapuram S.S.: 230KV C.B. Control Cubicle To Control Panel (201RB)Document2 pages230KV Line Feeder at 230/ 110KV Savasapuram S.S.: 230KV C.B. Control Cubicle To Control Panel (201RB)Siemens Rmu100% (1)
- Formulas For Perimeter, Area, and Volume: 5 2l 5 2w 5 LW 5 4s 5 SDocument1 pageFormulas For Perimeter, Area, and Volume: 5 2l 5 2w 5 LW 5 4s 5 SRediet UtteNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- Solidworks MockUp DrawingDocument7 pagesSolidworks MockUp DrawingPANDU RADITYANo ratings yet
- Electrical and Electronic Principles 3 Checkbook: The Checkbook SeriesFrom EverandElectrical and Electronic Principles 3 Checkbook: The Checkbook SeriesNo ratings yet
- Lista de Exercicios Sobre AlcanosDocument4 pagesLista de Exercicios Sobre AlcanosquelfisicaNo ratings yet
- Class: Xi Inorganic Chemistry DPP. NO.-9Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-9Radhika MohataNo ratings yet
- Organic - Class 7Document27 pagesOrganic - Class 7Sajan Singh LUCKYNo ratings yet
- Teori Ikatan Valensi, Hibridisasi, Dan VSEPRDocument43 pagesTeori Ikatan Valensi, Hibridisasi, Dan VSEPRSisca Ayu VerawatiNo ratings yet
- 05 StereoIsomerismDocument26 pages05 StereoIsomerismAnweshNo ratings yet
- PPTDocument31 pagesPPTtessyNo ratings yet
- 3 Bonding Pairs: Exercise No. 3 Lewis Structure and Molecular GeometryDocument2 pages3 Bonding Pairs: Exercise No. 3 Lewis Structure and Molecular GeometryClaireNo ratings yet
- Advanced Practice Test Aceg 1. (A) (B) (C) (D) 2. (A) (B) (C) (D) 3. (A) (B) (C) (D) 4. (A) (B) (C) (D) 5. (A) (B) (C) (D)Document7 pagesAdvanced Practice Test Aceg 1. (A) (B) (C) (D) 2. (A) (B) (C) (D) 3. (A) (B) (C) (D) 4. (A) (B) (C) (D) 5. (A) (B) (C) (D)ANCHIT JAIN QUESTION PAPERSNo ratings yet
- EVAN BLIZZARD - 20-21 PhET Simulation - Molecular GeometryDocument7 pagesEVAN BLIZZARD - 20-21 PhET Simulation - Molecular GeometryEVAN BLIZZARDNo ratings yet
- Moon - Exam 2 - Summer 2011Document10 pagesMoon - Exam 2 - Summer 2011Andres Pena100% (2)
- Lec 1 - MCDocument15 pagesLec 1 - MCDivyam JainNo ratings yet
- Conformations of Alkanes and CycloalkanesDocument9 pagesConformations of Alkanes and CycloalkanesFakhrul RaziNo ratings yet
- Conformations of Alkanes and CycloalkanesDocument18 pagesConformations of Alkanes and CycloalkanesjuanNo ratings yet
- The Significance of Chirality in Drug Design and DevelopmentDocument11 pagesThe Significance of Chirality in Drug Design and DevelopmentBrahma Hakim Yuanda HutabaratNo ratings yet
- Optical Isomerism NotesDocument5 pagesOptical Isomerism NotesTemi BalogunNo ratings yet
- 2 AlkanesDocument53 pages2 Alkaneskitlung wanNo ratings yet
- Stereochemistry A Three Dimensional Insight Anil V Karnik and Mohammed Hasan Full Download ChapterDocument51 pagesStereochemistry A Three Dimensional Insight Anil V Karnik and Mohammed Hasan Full Download Chaptersherri.logan960100% (5)
- Stereochemistry Sem.Document6 pagesStereochemistry Sem.Muhammad AalimNo ratings yet
- Single Choice Question 1.: H CH CH H H CH CH CH H CH CH CH H CHDocument7 pagesSingle Choice Question 1.: H CH CH H H CH CH CH H CH CH CH H CHSumit RajNo ratings yet
- CHEM 210 CH05 StereochemistryDocument10 pagesCHEM 210 CH05 StereochemistryJennifer MaamaryNo ratings yet
- Stereochemistry & Reaction MechanismDocument32 pagesStereochemistry & Reaction MechanismPranjal KumarNo ratings yet
- Vsepr HandoutDocument2 pagesVsepr HandoutAdrianne Jericho ValdezNo ratings yet
- Isomerism KEC 079 Lecture III BCE A 079-02-10Document13 pagesIsomerism KEC 079 Lecture III BCE A 079-02-10bsarad115No ratings yet
- Unit I PCPDocument60 pagesUnit I PCPsuranjana26No ratings yet
- CHEM 210 Chapter 5 Wrap-UpDocument27 pagesCHEM 210 Chapter 5 Wrap-UpTuan NguyenNo ratings yet
- CHM 1321 Assignment 3 - : AnswersDocument5 pagesCHM 1321 Assignment 3 - : AnswersSara YuenNo ratings yet
- Stereochemistry MSCDocument29 pagesStereochemistry MSCBapu Thorat50% (2)
- Understanding Fischer ProjectionsDocument3 pagesUnderstanding Fischer Projectionspfeffer92No ratings yet
- CY1101 (Autumn-2021-2022) - Class Test (Organic Section) 11.03.2022 (Preview) Microsoft FormsDocument17 pagesCY1101 (Autumn-2021-2022) - Class Test (Organic Section) 11.03.2022 (Preview) Microsoft FormsAdarsh PriyaranjanNo ratings yet
- Stereochemistry Notes For CHM 102Document4 pagesStereochemistry Notes For CHM 102faborodeharyomideNo ratings yet