Professional Documents
Culture Documents
Gonadal
Gonadal
Uploaded by
Jolan Fernando HerceCopyright:
Available Formats
You might also like
- L25 PDFDocument46 pagesL25 PDFMiles HuiNo ratings yet
- Retinoblastoma Case StudyDocument138 pagesRetinoblastoma Case Studyallexiscampaner100% (4)
- Sistem Reproduksi Pria (DR - Ekop)Document56 pagesSistem Reproduksi Pria (DR - Ekop)Laras OktavianiNo ratings yet
- Lecture 45:endocrine Functions of The Testes: DR ShamshadDocument31 pagesLecture 45:endocrine Functions of The Testes: DR ShamshadShivani RajNo ratings yet
- Chapter 125Document18 pagesChapter 125Saloni SaloniNo ratings yet
- Male Reproductive HormonesDocument29 pagesMale Reproductive HormonesAjit ShahNo ratings yet
- Lecture 9 Human AnatomyDocument4 pagesLecture 9 Human AnatomyAngelica CruzNo ratings yet
- Male ReproductiveDocument10 pagesMale ReproductivevivekNo ratings yet
- Sistem Reproduksi PriaDocument67 pagesSistem Reproduksi PriaMuthi'ah TsamarahNo ratings yet
- EndocrineDocument4 pagesEndocrineMary Kaye Yvonne OtillaNo ratings yet
- Assignment of Physiology IIDocument21 pagesAssignment of Physiology IInejaabera12No ratings yet
- TestesDocument15 pagesTestesTem GemechuNo ratings yet
- Grade 10 Biology ReviewerDocument10 pagesGrade 10 Biology ReviewerNadine Victoria DizonNo ratings yet
- Adi Androgel Diagnosis and Testosterone Replacement Therapy - RTD Slide 2020Document43 pagesAdi Androgel Diagnosis and Testosterone Replacement Therapy - RTD Slide 2020Rima EffendiNo ratings yet
- Hormones and It's TypesDocument10 pagesHormones and It's TypesSidraNo ratings yet
- Mawhinney Et Al-2013-Periodontology 2000Document20 pagesMawhinney Et Al-2013-Periodontology 2000Dr. DeeptiNo ratings yet
- CC22Document20 pagesCC22Kathlyn Abby HaliliNo ratings yet
- Anfis TestisDocument4 pagesAnfis TestisNandia SeptiyoriniNo ratings yet
- Endocrine SystemDocument4 pagesEndocrine SystemJames Castro100% (1)
- Page From G-K-Pal-Comprehensive-Textbook-of-Medical-PhysiologyDocument1 pagePage From G-K-Pal-Comprehensive-Textbook-of-Medical-Physiologysingonstrings365No ratings yet
- Spermatogenesisvs OogenesisDocument96 pagesSpermatogenesisvs OogenesisZerica JohnNo ratings yet
- AndrologyDocument57 pagesAndrologyaginche amareNo ratings yet
- Physiology of Reproductive SystemDocument25 pagesPhysiology of Reproductive SystemEstherNo ratings yet
- 10 Endocrine SystemDocument3 pages10 Endocrine SystemMardy Martin SorianoNo ratings yet
- Anaphy SemiDocument14 pagesAnaphy SemipersonalimouNo ratings yet
- Reproduction Student MaterialDocument27 pagesReproduction Student MaterialEsosa OdighizuwaNo ratings yet
- Endocrine System - HANDOUTSDocument20 pagesEndocrine System - HANDOUTSMeegs EstabilloNo ratings yet
- Male Reproductive PhysiologyDocument83 pagesMale Reproductive PhysiologyX Christian CatalanNo ratings yet
- Gynecology Review: Abigail Elsie Dg. Castro, MD, Maed, Fpogs, Fpsuog August 11, 2017Document198 pagesGynecology Review: Abigail Elsie Dg. Castro, MD, Maed, Fpogs, Fpsuog August 11, 2017Jojo Mendoza100% (1)
- 9 Male Sex HormonesDocument22 pages9 Male Sex HormonesRana AbdullahNo ratings yet
- Male Reproductive PhysiologyDocument18 pagesMale Reproductive Physiologykushluv38No ratings yet
- Sex Determination ReportDocument35 pagesSex Determination ReportCarmela LipoNo ratings yet
- Hormonal Management of Male InfertilityDocument22 pagesHormonal Management of Male Infertilitydranibalurologoencancun.mxNo ratings yet
- Endocrine SystemDocument7 pagesEndocrine SystemMikaella Viador100% (2)
- Endocrinesys TEM: Principles of Chemical CommunicationDocument4 pagesEndocrinesys TEM: Principles of Chemical CommunicationCatherine Joy delos SantosNo ratings yet
- Basics of Endocrinology: Kathleen Colleran MD Associate Professor of MedicineDocument48 pagesBasics of Endocrinology: Kathleen Colleran MD Associate Professor of MedicineClaudia IrimieNo ratings yet
- Course Number: HSC 1201 Course Title: Homeostasis 11Document33 pagesCourse Number: HSC 1201 Course Title: Homeostasis 11Aneesa A HenryNo ratings yet
- EnglishDocument10 pagesEnglisharthurggg429No ratings yet
- RS-1 K5-K6 Hormon Repro Pria Dan Wanita, EditDocument57 pagesRS-1 K5-K6 Hormon Repro Pria Dan Wanita, EditfelixNo ratings yet
- PPPPPPPTTTTTTTTTTTTTTDocument16 pagesPPPPPPPTTTTTTTTTTTTTTfaisal_gondal786No ratings yet
- Mind Map Chemical Control and Integration Final ReviewedDocument78 pagesMind Map Chemical Control and Integration Final Reviewed09 Krishna TrivediNo ratings yet
- Reproductive PhysiologyDocument38 pagesReproductive Physiologysam bossaNo ratings yet
- ReproductionDocument31 pagesReproductionAbebe SetiyeNo ratings yet
- Lecture Reproductive - Glands FinalDocument32 pagesLecture Reproductive - Glands FinalerikaquitaraaaNo ratings yet
- Male Reproductive Function Sept 2014Document32 pagesMale Reproductive Function Sept 2014Nirmesh ThanabalanNo ratings yet
- Chemical Co Ordination.Document11 pagesChemical Co Ordination.PAK BTS ARMYNo ratings yet
- Midterm - AnaphyDocument6 pagesMidterm - AnaphyJustine Mae OyongNo ratings yet
- Chemical Coordination and IntegrationDocument3 pagesChemical Coordination and Integrationakhil01ajNo ratings yet
- Chapter 10 ANATOMY AND PHYSIOLOGYDocument5 pagesChapter 10 ANATOMY AND PHYSIOLOGYAngela Mae MeriñoNo ratings yet
- Kuliah Patologi EndokrinDocument24 pagesKuliah Patologi Endokrinrizki ardiansyahNo ratings yet
- Share Endokrinologi Reproduksi PriaDocument24 pagesShare Endokrinologi Reproduksi PriaBrama AtmajaNo ratings yet
- Male Reproduction SystemDocument22 pagesMale Reproduction SystemSalman KhanNo ratings yet
- Gametogenesis: Spermatogenesis Dan OogenesisDocument74 pagesGametogenesis: Spermatogenesis Dan OogenesisZidny FuraidahNo ratings yet
- Gynaecology Project1Document15 pagesGynaecology Project1instagramfollowers168No ratings yet
- Androgens and Related Drugs, Drugs For Erectile DysfunctionDocument52 pagesAndrogens and Related Drugs, Drugs For Erectile Dysfunctionayushnaithani004No ratings yet
- Male Reproductive SystemDocument26 pagesMale Reproductive SystemEmmanuelNo ratings yet
- Hormone Action in The Mammary Gland - C Brisken and B O Malley 2010Document15 pagesHormone Action in The Mammary Gland - C Brisken and B O Malley 2010tostonasNo ratings yet
- Bp503t Pcol Unit-VDocument46 pagesBp503t Pcol Unit-VAakkkNo ratings yet
- Tutorial 4.5Document4 pagesTutorial 4.5Rosa FinizioNo ratings yet
- Endocrine System 3 Adrenal Gland, Testes and OvariesDocument73 pagesEndocrine System 3 Adrenal Gland, Testes and OvariesdeeznutsnikkaballzNo ratings yet
- Endocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceFrom EverandEndocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceNo ratings yet
- Background of The ProjectDocument2 pagesBackground of The ProjectJolan Fernando HerceNo ratings yet
- Homework 1 Herce Bsmt2bDocument1 pageHomework 1 Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Biochemistry Herce Bsmt2bDocument1 pageBiochemistry Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Biochem Herce Bsmt2bDocument3 pagesBiochem Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Activity 1 Herce Bsmt1bDocument1 pageActivity 1 Herce Bsmt1bJolan Fernando HerceNo ratings yet
- Assessment QuestionnaireDocument3 pagesAssessment QuestionnaireJolan Fernando HerceNo ratings yet
- APznzab7n0tTt9B3o6nrEulLujxEm FmBRigdivDocument2 pagesAPznzab7n0tTt9B3o6nrEulLujxEm FmBRigdivJolan Fernando HerceNo ratings yet
- Activity On Endocrinology HERCE BSMT1BDocument1 pageActivity On Endocrinology HERCE BSMT1BJolan Fernando HerceNo ratings yet
- 20 Amino Acids Herce Bsmt2bDocument2 pages20 Amino Acids Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Letter of Two Students For InterviewDocument1 pageLetter of Two Students For InterviewJolan Fernando HerceNo ratings yet
- Cytogen FnalsDocument17 pagesCytogen FnalsJolan Fernando HerceNo ratings yet
- SEATWORK1Document2 pagesSEATWORK1Jolan Fernando HerceNo ratings yet
- ACFrOgBSxI6Havvmnh9Te1qpS qBO9uYA HesicDocument6 pagesACFrOgBSxI6Havvmnh9Te1qpS qBO9uYA HesicJolan Fernando HerceNo ratings yet
- Covid19 Pandemic - HerceDocument6 pagesCovid19 Pandemic - HerceJolan Fernando HerceNo ratings yet
- Practical Test-Balancing and Types of Chemical RXNDocument1 pagePractical Test-Balancing and Types of Chemical RXNJolan Fernando HerceNo ratings yet
- Group Learning ActivityDocument3 pagesGroup Learning ActivityJolan Fernando HerceNo ratings yet
- Script For StepsDocument5 pagesScript For StepsJolan Fernando HerceNo ratings yet
- Medical Quackery and Folk MedicinesDocument2 pagesMedical Quackery and Folk MedicinesJolan Fernando HerceNo ratings yet
- Steps in Foodborne OutbreakDocument2 pagesSteps in Foodborne OutbreakJolan Fernando HerceNo ratings yet
- Biostat and Epi Transes SophomoresDocument60 pagesBiostat and Epi Transes SophomoresJolan Fernando HerceNo ratings yet
- Activity 8 - HERCE - BSMT1BDocument3 pagesActivity 8 - HERCE - BSMT1BJolan Fernando HerceNo ratings yet
- Activity Lab Sheet 4Document5 pagesActivity Lab Sheet 4Jolan Fernando HerceNo ratings yet
- Cytogen ReviewerDocument6 pagesCytogen ReviewerJolan Fernando HerceNo ratings yet
- QajjquoisiDocument41 pagesQajjquoisiJolan Fernando HerceNo ratings yet
- Mammo Prelim ReviewerDocument17 pagesMammo Prelim ReviewerCherie lou PizaNo ratings yet
- Stomac Și Duoden - LawrenceDocument45 pagesStomac Și Duoden - LawrenceNicole IoanidNo ratings yet
- Regulation of Cardiac Output, Intrinsic and Extrinsic FactorsDocument49 pagesRegulation of Cardiac Output, Intrinsic and Extrinsic FactorsPeter Mukunza100% (1)
- NHMRC Research Achievements - SUMMARY: Funded Research Into Injury Related Research ENDING 2004 TO 2013Document521 pagesNHMRC Research Achievements - SUMMARY: Funded Research Into Injury Related Research ENDING 2004 TO 2013Titoun LettounNo ratings yet
- Tera P90Document20 pagesTera P90samuel2koffiNo ratings yet
- Abdomen OSPE Charts Questions OnlyDocument29 pagesAbdomen OSPE Charts Questions OnlyRathnaNo ratings yet
- MER TopicsDocument4 pagesMER TopicsYaminiNo ratings yet
- Glaukoma Sekunder Pada Pasien Anak Dengan Sindrom Sturge Weber: Laporan KasusDocument7 pagesGlaukoma Sekunder Pada Pasien Anak Dengan Sindrom Sturge Weber: Laporan KasusLordisse WongNo ratings yet
- Tong Ren Poster - Janice GoldmanDocument1 pageTong Ren Poster - Janice Goldmanphani kumarNo ratings yet
- 組 員: 賴 冠 穎 U 1 0 7 S 1 4 6 游 育 瓏 U 1 0 8 0 2 0 1 洪 旻 謙 U 1 0 8 0 2 1 0 楊 易 達 U 1 0 8 0 2 5 9 簡 佳 瑋 U 1 0 7 0 1 6 4 謝 文 予 U 1 0 7 0 1 3 9Document13 pages組 員: 賴 冠 穎 U 1 0 7 S 1 4 6 游 育 瓏 U 1 0 8 0 2 0 1 洪 旻 謙 U 1 0 8 0 2 1 0 楊 易 達 U 1 0 8 0 2 5 9 簡 佳 瑋 U 1 0 7 0 1 6 4 謝 文 予 U 1 0 7 0 1 3 9William ChienNo ratings yet
- Human Memory 2nd Radvansky Test BankDocument9 pagesHuman Memory 2nd Radvansky Test BankJoshua Warren100% (38)
- Q4 - Module 3 Consumer ChemistryDocument23 pagesQ4 - Module 3 Consumer Chemistryjessie100% (2)
- Surgical Notes (Teddy)Document88 pagesSurgical Notes (Teddy)shichianNo ratings yet
- Self-Resolving Ischemic Third Nerve PalsyDocument10 pagesSelf-Resolving Ischemic Third Nerve PalsyYobbi ArisaputraNo ratings yet
- The Developing BrainDocument11 pagesThe Developing BrainRhodaNo ratings yet
- NCM 112 - Cardio 2 - Advance StudyDocument2 pagesNCM 112 - Cardio 2 - Advance StudyUzumaki KNo ratings yet
- Endocrine System: MSN 4006 Advanced PsychopathophysiologyDocument15 pagesEndocrine System: MSN 4006 Advanced Psychopathophysiologybane1925No ratings yet
- The Nervous System: EssentialsDocument27 pagesThe Nervous System: EssentialsSunshine LabraNo ratings yet
- Biomedicine & Pharmacotherapy: Original ArticleDocument8 pagesBiomedicine & Pharmacotherapy: Original ArticleYasmeen AbassNo ratings yet
- ReproductionDocument95 pagesReproductionSalman KhanNo ratings yet
- Seizure: Dr. Muhammad Yusuf, SP.S, FINSDocument24 pagesSeizure: Dr. Muhammad Yusuf, SP.S, FINSGina SoniaNo ratings yet
- Neurological DisordersDocument11 pagesNeurological DisordersFatima AsimNo ratings yet
- Hsslive Xi Zoology Rev Test 6 QN by Zta Malappuram - 230212 - 110004 PDFDocument4 pagesHsslive Xi Zoology Rev Test 6 QN by Zta Malappuram - 230212 - 110004 PDFഎസ്സാർപി ഊരുചുറ്റൽ തുടരുന്നുNo ratings yet
- Radiographic Features of RL LesionsDocument7 pagesRadiographic Features of RL Lesionszyad ahmedNo ratings yet
- 1 Klöppel Vortrag GEP NET The NE System 120217Document32 pages1 Klöppel Vortrag GEP NET The NE System 120217soledad88No ratings yet
- Gas 053Document11 pagesGas 053Angham OuadahNo ratings yet
- Small Animal Neurological Emergencies PLATTDocument673 pagesSmall Animal Neurological Emergencies PLATTrebeca garay100% (2)
- FTEC LESSON 34 Meat TechnologyDocument30 pagesFTEC LESSON 34 Meat Technologyjennelyn.antayNo ratings yet
- Burn Mmii - MMSS PDFDocument24 pagesBurn Mmii - MMSS PDFLuis A De la CubaNo ratings yet
Gonadal
Gonadal
Uploaded by
Jolan Fernando HerceOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gonadal
Gonadal
Uploaded by
Jolan Fernando HerceCopyright:
Available Formats
CLINICAL CHEMISTRY Jolan F.
Herce
BSMT-3B
LECTURE / 2nd SEMESTER
• LH: affects Leydig cells in the testicular insterstitium
• Gonadotropins: glycoproteins with an alpha subunit and a beta
CHAPTER 22: Gonadal Function
subunit
THE TESTES
• Primordial germ cells migrate to the gonadal ridges, forming
primitive gonads.
• The gonad is bipotential, either 46 XX or 46 XY. Hormonal Control of Testicular Function
• In the presence of the Y chromosome, medullary tissue forms testes • Gonadotropin-Releasing Hormone (GnRH): produced in the
with seminiferous tubules and Leydig cells, capable of androgen hypothalamus and released into the portal hypophyseal system
secretion. Impaired GnRH generation leads to hypogonadism.
• Spermatogonia: derived from primordial germ cells, forms the walls • Cholesterol conversion to pregnenolone is the first step in testicular
of the seminiferous tubules. steroidogenesis.
• Leydig cells: produce androgen, stimulating mesonephric ducts to • Testosterone and inhibin provide feedback control to the
form male genital ducts. hypothalamus and pituitary.
• Three steps in sexual differentiation and development of the • Testosterone is the principal androgen hormone in the blood, and
normal male phenotype: after puberty the testes secrete 4 to 10 mg daily and less than 5% is
1. differentiation of bipotential gonad primordia into testes that derived from adrenal precursors such as dehydroepiandrosterone
secrete testosterone (DHEA) and androstenedione.
2. development of the internal reproductive tract • Leydig cell steroidogenesis of testosterone: primarily controlled by
3. development of external genitalia that require testosterone LH secretion
and its more potent metabolite 5α-dihydrotestosterone (DHT) • FSH acts on Sertoli cells to stimulate protein synthesis, inhibin, and
• Three genes involved in gonadal differentiation: androgen-binding protein.
1. WT11 Serum levels of testosterone:
2. LIM12 ↑ 6 am
3. FTZ-F1 ↓ 12 am
Functional Anatomy of the Male Reproductive Tract
Adult testes - paired, ovoid organs that hang from the Cellular Mechanism of Testosterone Action
inguinal canal by the spermatic cord, which comprises • Testosterone enters cells, converts to DHT in 5α-reductase-rich
neurovascular pedicle cells.
vas deferens • Complex binds to nuclear receptor, affecting protein synthesis and
cremasteric muscle cell growth.
Location: outside the body, encased by a muscular sac
• Blood flow regulates the temperature of the testicles to 2°C below
core body temperature, crucial for uninterrupted sperm production. Physiologic Actions of Testosterone
Two anatomical units: Prenatal Development
1. network of tubules - seminiferous tubules • Primitive gonads become distinguishable at the 7 th week of embryonic
2. interstitium - tubules contain germ cells and Sertoli cells and stage.
are responsible for sperm production • Chorionic gonadotropins and fetal LH stimulate testosterone
Functions of Testes: production by fetal Leydig cells.
1. production of sperm • Hypothalamohypophyseal vascular connections in fetus release LH
2. production of reproductive steroid hormones between 11 and 12 weeks after conception.
• Puberty marks the transition from a non-reproductive • hCG, with LH-like action: accounts for testosterone production gap
state into a reproductive state and is associated with • Testosterone exposure to the Wolffian duct differentiates male
adrenarche with increase in adrenal androgen and gonadarche. genital tract components.
Earliest sign of puberty: testicular enlargement that results from ↑ • Sertoli cells produce AMH for female primordial genital tract
luteinizing hormone (LH) and follicle-stimulating hormone (FSH) regression.
Seminal vesicle secretions: rich in vitamin C and fructose (important • Scrotal skin's 5α-reductase converts testosterone to DHT.
for the preservation of motility of the sperm) • Drugs blocking this enzyme and DHT formation lead to male fetus
genitalia feminization.
Physiology of the Testicles
Spermatogenesis
• undergoes mitosis and meiosis
• Haplotype cells: transform into mature sperm
• Mature sperm: has a head, body, and tail for zygote formation.
• Certain spermatogonia stagger division for uninterrupted sperm
production.
• Sertoli cells: aid in sperm development and maturation.
Hormonogenesis
• Testosterone, the main hormone secreted by the testes, is regulated
by FSH and LH.
• Named after the menstrual cycle, these hormones are produced by
gonadotrophs.
• FSH: affects germinal stem cells.
TRANSCRIBED BY: JOLAN HERCE
CLINICAL CHEMISTRY Jolan F. Herce
BSMT-3B
LECTURE / 2nd SEMESTER
Postnatal Development • an autosomal dominant condition
• characterized by: primary hypogonadism, frontal balding,
diabetes, muscle weakness, atrophy, and dystonia, often presenting
in the fourth decade of life
Testicular Injury and Infection
• Post-pubertal mumps infection can cause mumps orchitis,
permanent testicular injury, viral orchitis, HIV infection, and long-
term damage from radiation and chemotherapy for cancer.
Sertoli cell-only syndrome
• condition where men lack germ cells, presenting with small testes,
high FSH levels, azoospermia, and normal testosterone levels,
confirmed by a testicular biopsy
HYPOGONADOTROPIC HYPOGONADISM
Effect on Spermatogenesis occurrence of low testosterone levels together with low or
• Leydig cell stimulation triggers testosterone production, which, when inappropriately normal FSH or LH levels
combined with FSH, stimulates seminiferous and Sertoli cells,
promoting spermatogenesis. Kallmann’s Syndrome
• X-linked form of congenital GnRH deficiency
Effect on Secondary Sexual Effects • impairs GnRH secretion due to impaired migration of neurons
• Testosterone promotes growth and maintains secondary sexual • Mutations in the KAL1 gene, found on the X chromosome, cause
characteristics in adulthood, leading to prostate enlargement and idiopathic hypogonadotropic hypogonadism and Hallmann's
hairline recession. syndrome
• Low testosterone levels can cause bone mass loss and osteoporosis.
Disorders of Sexual Development and Testicular Hypofunction
HYPERGONADOTROPIC HYPOGONADISM
incorporates a group of disorders characterized by low
testosterone, elevated FSH or LH, and impaired sperm
production
Klinefelter’s Syndrome
• about 1 of 400 men due to an extra chromosome; common
karyotype is 47,XY.
• Men with this disorder have small (<2.5cm), firm testicles.
• Gynecomastia (enlargement of the male breast) can also be
present
• ↓ testosterone, ↑ FSH and LH
• These men also have azoospermia and resultant sterility.
• Men with mosaicism may produce some sperm and pregnancies
have been reported
Testicular Feminization Syndrome
• Severe form of androgen resistance syndrome causing lack of
testosterone action in target tissue.
• Physical development pursues female phenotype with fully
developed breast and female fat and hair distribution.
• Most men present for primary amenorrhea evaluation, revealing
lack of female internal genitalia.
• Biochemical evaluation shows N testosterone levels,
↑ FSH and LH levels, no response to exogenous testosterone.
5α-reductase deficiency
• causes androgen insensitivity in XY males, affecting DHT
concentrations
• causing physical development similar to females until puberty,
affecting male internal genitalia
Myotonic dystrophy
TRANSCRIBED BY: JOLAN HERCE
CLINICAL CHEMISTRY Jolan F. Herce
BSMT-3B
LECTURE / 2nd SEMESTER
Hyperprolactinemia • Dosage based on lean body mass, not weight.
Prolactin elevation from drug-induced or prolactin-producing • Aim: Maintain mid-dose level at normal ranges.
pituitary tumors can lead to hypogonadotropic hypogonadism due to
impaired pulsatile secretion of FSH and LH, possibly disrupting 2. Transdermal testosterone patch therapy
GnRH pulsatile secretion. • Provides physiologic testosterone levels.
Type 2 Diabetes • Enhances skin permeability for absorption.
Hypogonadotropic hypogonadism, a condition characterized by low • May cause local skin irritation.
testosterone concentrations and inappropriate low LH, is linked to
insulin resistance and inflammation in at least 25% of males with 3. Testosterone gel
type 2 diabetes. • Daily application to non-genital skin.
Age • Gradual absorption.
Testosterone levels gradually decrease after age 30, with an • Provides normal testosterone levels for 24 hours.
average decline of 110 ng/dL every decade. Age is associated with • Concerns: potential transmission to female partners or children
increased SHBG and bioavailable testosterone levels. Testosterone
deficiency, combined with hypogonadism and low serum 4. Testosterone buccal pellet
testosterone concentrations, prompts testosterone replacement • Placed twice daily.
consideration. • Local discomfort.
Pituitary Disease • Twice-daily dosing limits usage.
• Acquired hypogonadism due to tumors, surgical trauma, vascular
injury, autoimmune hypophysitis, or granulomatous/metastatic
disease. 5. Testosterone pellet
• Rare cause: Hemochromatosis • Recommends three to six 75 mg testosterone pellets every 3 to 6
months.
• Implanted into subdermal fat of buttocks, lower abdominal wall, or
Diagnosis of Hypogonadism thigh.
• Risks: pellet extrusion, infection, and fibrosis.
• Limited data on serum testosterone concentrations limits routine use.
Monitoring Testosterone Replacement Therapy
Regularly check prostate-specific antigen (PSA), blood counts, and
lipid levels after testosterone replacement, and consider clinical
evaluation for leg edema, sleep apnea, and prostate enlargement.
THE OVARIES
Early Ovarian Development
• The gonad forms an ovary without a Y chromosome or TDF.
• The cortex develops, the medulla regresses, and oogonia develop
within follicles.
• Oogonia: from primitive germ cells by a series of about 30 mitoses
o enter meiosis I at the end of the third month
o arrested at dictyotene stage
• Only about 400 mature into ova during the 30 years of female sexual
maturity.
• Estrogen formation in the fetal ovary begins early development.
• Gonadotropins from the pituitary gradually take over the role of
maternal placental hCG.
• Fetal pituitary LH and FSH peak near mid-gestation and fall to low
concentrations at birth.
• Postpartum, a smaller peak of LH and FSH occurs, stimulating
steroid secretion and neonatal milk production.
Pubertal Changes of Ovarian Function
• ↑ secretion of LH and FSH stimulates gonadal activity
Testosterone Replacement Therapy • Ovaries perform gamete and steroid hormone production.
Testosterone should be administered only to a man who is • Menstrual cycle, 28 days, prepares uterus for embryo implantation.
Hypogonadal.
The currently available modes of testosterone administration in the Functional Anatomy of the Ovaries
United States are as follows: • Ovaries are oval organs located in the pelvic fossa, attached to the
1. Parenteral testosterone broad ligament by the mesovarium.
• Cypionate and enanthate esters of testosterone available for • Adult ovaries are 2 to 5 cm long, weigh 14 g, and contain 2 to 4
intramuscular injection. million primordial follicles.
• Peak testosterone level achieved in 72 hours, lasting 1 to 2 weeks. • Each ovarian cycle involves recruitment of a few primordial follicles
• Weekly administration for lower peak and less fluctuation. for maturation.
• Dosing range: 50 to 100 mg weekly or 200 to 250 mg once every 2 • Graafian follicle (single remaining follicle) is composed of an outer
weeks. and inner layer, a central cavity, and a granulosa layer
TRANSCRIBED BY: JOLAN HERCE
CLINICAL CHEMISTRY Jolan F. Herce
BSMT-3B
LECTURE / 2nd SEMESTER
o ovum attaches to the follicle via cumulus cells derived from Hormonal Control of Ovulation
granulosa cells • Controls FSH and LH secretion.
o releases its ovum in response to ovarian stimulation by LH • Rises estrogen levels post-menopause.
• Corpus luteum: rich in cholesterol, acts as a substrate for • Elevated FSH levels in follicular phase.
progesterone and estrogen production, maintaining the endometrium • Midcycle surges in LH production trigger ovulation.
for conception.
Pubertal Development in the Female
• Involves hormonal events.
• Leads to secondary sexual characteristics and adult weight.
Hormonal Production by the Ovaries • Thelarche, breast tissue development, pubic hair growth, menarche.
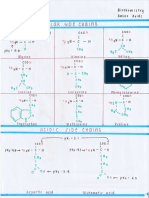
Estrogen
carbon-18 compounds
primarily produced in the ovary Precocious Sexual Development
play a crucial role in breast, uterine, and vaginal development,
affecting various organs during reproductive periods
Menstrual Cycle Abnormalities
Progesterone
• 25 to 35 days
carbon-21 compound • average 28-day duration.
produced by the corpus luteum and induces endometrial gland Amenorrhea
secretion preparing the endometrium for embryo implantation o absence of menses
thickens cervical mucus, reduces uterine contractions, and has a
thermogenic effect Type 1. Hypothalamic hypogonadism (low or normal
FSH and/or LH)
Androgens Hypothalamic amenorrhea (anorexia nervosa, idiopathic,
androstenedione, testosterone, and DHT exercise induced)
o can lead to hair growth, female characteristics loss, and Kallmann’s syndrome
masculinization in severe cases Isolated gonadotropin deficiency
Type 2. Estrogenic chronic anovulation (normal FSH
Others and LH)
Inhibins A and B inhibit FSH production Polycystic ovarian syndrome (LH > FSH in some patients 2:1
Activin enhances FSH secretion and induces steroidogenesis. or greater)
Folliculostatin, relaxin, follicle regulatory protein, oocyte Hyperthecosis
maturation factor, and meiosis inducing substance have
unspecified functions. Type 3. Hyperthalamic hypogonadism (elevated FSH and/or LSH)
Premature ovarian failure
The Menstrual Cycle Turner’s syndrome
The Follicular Phase
• Beginning with menstruation.
• Ending with LH surge. Primary amenorrhea
• Ovarian secretes minimal estrogen/progesterone. o woman has never menstruated
• Enhances uterine epithelial cells and endometrial gland Secondary amenorrhea
development. o woman who has had at least one menstrual cycle
followed by absences of menses for a minimum of 3–6
The Luteal Phase months.
• Estrogen levels peak 1 day before ovulation, triggering LH surge. Oligomenorrhea
• LH phase begins with ovulation extrusion and luteinization of o irregular menstrual bleeding
Graafian follicle. o cycle lengths in excess of 35–40 days.
• Corpus luteum secretes progesterone for embryo implantation.
Menorrhagia
• Without fertilization, endometrial blood supply declines, leading to
o Uterine bleeding in excess of 7 days and is considered
shedding 14 days post-ovulation.
dysfunctional
• Menstrual bleeding lasts 3-5 days.
HYPOGONADOTROPIC HYPOGONADISM
• Deficiency in gonadotropin (FSH and LH) leading to decreased sex
steroid production.
• Common cause of secondary amenorrhea.
• Causes: weight loss, intense exercise, pituitary tumors, and
prolactinomas
HYPERGONADOTROPIC HYPOGONADISM
• Characterized by ovarian failure leading to FSH and LH elevations.
TRANSCRIBED BY: JOLAN HERCE
CLINICAL CHEMISTRY Jolan F. Herce
BSMT-3B
LECTURE / 2nd SEMESTER
• Ovarian failure occurs naturally between 45-55 years in American
women.
• Menopause, a natural event, results in elevated FSH and LH levels
with low estrogen levels.
• Premature ovarian failure is primary hypogonadism before 40,
resulting from congenital chromosomal abnormality or premature
menopause.
• Patients with Turner’s syndrome do not experience the same hot
flashes as those with secondary hypergonadotropic hypogonadism.
• Premature menopause can occur alone or in conjunction with other
endocrine gland failures.
Polycystic Ovary Syndrome
• Characterized by: infertility, hirsutism, hypertension
• Diagnosis: ovarian ultrasound
• Most patients are overweight, some normal.
• Symptoms reversed with weight loss and increased activity.
• Glucophage, diabetes treatment drug, normalizes menstrual cycles
and improves conception rates.
Hirsutism
CLASSIFICATION OF HIRSUTISM
• Functional (normal androgen levels with excess hair growth) or
true androgen excess (elevated androgens)
• Ovarian (LH mediated) or adrenal (adrenocorticotropin hormone
mediated)
• Peripheral conversion of androgens (obesity)
• Tumoral hyperandrogenism (ovarian, adrenal)
• Chorionic gonadotropin mediated
CAUSES OF HIRSUTISM
COMMON
Idiopathic
Polycystic ovary syndrome
UNCOMMON
Drugs: danazol, oral contraceptives with androgenic
progestins
Congenital adrenal hyperplasia
Hyperprolactinemia
Cushing syndrome
Adrenal tumors
Ovarian tumors
Estrogen Replacement Therapy
• Women's Health Initiative study enrolled 16,608 postmenopausal
women.
• Found increased incidence of invasive breast cancer and venous clot
formation.
• No benefit in cognitive decline or coronary artery disease.
• Reductions in bone loss, colon polyp formation, and menopausal
symptoms noted.
• Currently, estrogen replacement is a treatment option after risk
counseling.
TRANSCRIBED BY: JOLAN HERCE
You might also like
- L25 PDFDocument46 pagesL25 PDFMiles HuiNo ratings yet
- Retinoblastoma Case StudyDocument138 pagesRetinoblastoma Case Studyallexiscampaner100% (4)
- Sistem Reproduksi Pria (DR - Ekop)Document56 pagesSistem Reproduksi Pria (DR - Ekop)Laras OktavianiNo ratings yet
- Lecture 45:endocrine Functions of The Testes: DR ShamshadDocument31 pagesLecture 45:endocrine Functions of The Testes: DR ShamshadShivani RajNo ratings yet
- Chapter 125Document18 pagesChapter 125Saloni SaloniNo ratings yet
- Male Reproductive HormonesDocument29 pagesMale Reproductive HormonesAjit ShahNo ratings yet
- Lecture 9 Human AnatomyDocument4 pagesLecture 9 Human AnatomyAngelica CruzNo ratings yet
- Male ReproductiveDocument10 pagesMale ReproductivevivekNo ratings yet
- Sistem Reproduksi PriaDocument67 pagesSistem Reproduksi PriaMuthi'ah TsamarahNo ratings yet
- EndocrineDocument4 pagesEndocrineMary Kaye Yvonne OtillaNo ratings yet
- Assignment of Physiology IIDocument21 pagesAssignment of Physiology IInejaabera12No ratings yet
- TestesDocument15 pagesTestesTem GemechuNo ratings yet
- Grade 10 Biology ReviewerDocument10 pagesGrade 10 Biology ReviewerNadine Victoria DizonNo ratings yet
- Adi Androgel Diagnosis and Testosterone Replacement Therapy - RTD Slide 2020Document43 pagesAdi Androgel Diagnosis and Testosterone Replacement Therapy - RTD Slide 2020Rima EffendiNo ratings yet
- Hormones and It's TypesDocument10 pagesHormones and It's TypesSidraNo ratings yet
- Mawhinney Et Al-2013-Periodontology 2000Document20 pagesMawhinney Et Al-2013-Periodontology 2000Dr. DeeptiNo ratings yet
- CC22Document20 pagesCC22Kathlyn Abby HaliliNo ratings yet
- Anfis TestisDocument4 pagesAnfis TestisNandia SeptiyoriniNo ratings yet
- Endocrine SystemDocument4 pagesEndocrine SystemJames Castro100% (1)
- Page From G-K-Pal-Comprehensive-Textbook-of-Medical-PhysiologyDocument1 pagePage From G-K-Pal-Comprehensive-Textbook-of-Medical-Physiologysingonstrings365No ratings yet
- Spermatogenesisvs OogenesisDocument96 pagesSpermatogenesisvs OogenesisZerica JohnNo ratings yet
- AndrologyDocument57 pagesAndrologyaginche amareNo ratings yet
- Physiology of Reproductive SystemDocument25 pagesPhysiology of Reproductive SystemEstherNo ratings yet
- 10 Endocrine SystemDocument3 pages10 Endocrine SystemMardy Martin SorianoNo ratings yet
- Anaphy SemiDocument14 pagesAnaphy SemipersonalimouNo ratings yet
- Reproduction Student MaterialDocument27 pagesReproduction Student MaterialEsosa OdighizuwaNo ratings yet
- Endocrine System - HANDOUTSDocument20 pagesEndocrine System - HANDOUTSMeegs EstabilloNo ratings yet
- Male Reproductive PhysiologyDocument83 pagesMale Reproductive PhysiologyX Christian CatalanNo ratings yet
- Gynecology Review: Abigail Elsie Dg. Castro, MD, Maed, Fpogs, Fpsuog August 11, 2017Document198 pagesGynecology Review: Abigail Elsie Dg. Castro, MD, Maed, Fpogs, Fpsuog August 11, 2017Jojo Mendoza100% (1)
- 9 Male Sex HormonesDocument22 pages9 Male Sex HormonesRana AbdullahNo ratings yet
- Male Reproductive PhysiologyDocument18 pagesMale Reproductive Physiologykushluv38No ratings yet
- Sex Determination ReportDocument35 pagesSex Determination ReportCarmela LipoNo ratings yet
- Hormonal Management of Male InfertilityDocument22 pagesHormonal Management of Male Infertilitydranibalurologoencancun.mxNo ratings yet
- Endocrine SystemDocument7 pagesEndocrine SystemMikaella Viador100% (2)
- Endocrinesys TEM: Principles of Chemical CommunicationDocument4 pagesEndocrinesys TEM: Principles of Chemical CommunicationCatherine Joy delos SantosNo ratings yet
- Basics of Endocrinology: Kathleen Colleran MD Associate Professor of MedicineDocument48 pagesBasics of Endocrinology: Kathleen Colleran MD Associate Professor of MedicineClaudia IrimieNo ratings yet
- Course Number: HSC 1201 Course Title: Homeostasis 11Document33 pagesCourse Number: HSC 1201 Course Title: Homeostasis 11Aneesa A HenryNo ratings yet
- EnglishDocument10 pagesEnglisharthurggg429No ratings yet
- RS-1 K5-K6 Hormon Repro Pria Dan Wanita, EditDocument57 pagesRS-1 K5-K6 Hormon Repro Pria Dan Wanita, EditfelixNo ratings yet
- PPPPPPPTTTTTTTTTTTTTTDocument16 pagesPPPPPPPTTTTTTTTTTTTTTfaisal_gondal786No ratings yet
- Mind Map Chemical Control and Integration Final ReviewedDocument78 pagesMind Map Chemical Control and Integration Final Reviewed09 Krishna TrivediNo ratings yet
- Reproductive PhysiologyDocument38 pagesReproductive Physiologysam bossaNo ratings yet
- ReproductionDocument31 pagesReproductionAbebe SetiyeNo ratings yet
- Lecture Reproductive - Glands FinalDocument32 pagesLecture Reproductive - Glands FinalerikaquitaraaaNo ratings yet
- Male Reproductive Function Sept 2014Document32 pagesMale Reproductive Function Sept 2014Nirmesh ThanabalanNo ratings yet
- Chemical Co Ordination.Document11 pagesChemical Co Ordination.PAK BTS ARMYNo ratings yet
- Midterm - AnaphyDocument6 pagesMidterm - AnaphyJustine Mae OyongNo ratings yet
- Chemical Coordination and IntegrationDocument3 pagesChemical Coordination and Integrationakhil01ajNo ratings yet
- Chapter 10 ANATOMY AND PHYSIOLOGYDocument5 pagesChapter 10 ANATOMY AND PHYSIOLOGYAngela Mae MeriñoNo ratings yet
- Kuliah Patologi EndokrinDocument24 pagesKuliah Patologi Endokrinrizki ardiansyahNo ratings yet
- Share Endokrinologi Reproduksi PriaDocument24 pagesShare Endokrinologi Reproduksi PriaBrama AtmajaNo ratings yet
- Male Reproduction SystemDocument22 pagesMale Reproduction SystemSalman KhanNo ratings yet
- Gametogenesis: Spermatogenesis Dan OogenesisDocument74 pagesGametogenesis: Spermatogenesis Dan OogenesisZidny FuraidahNo ratings yet
- Gynaecology Project1Document15 pagesGynaecology Project1instagramfollowers168No ratings yet
- Androgens and Related Drugs, Drugs For Erectile DysfunctionDocument52 pagesAndrogens and Related Drugs, Drugs For Erectile Dysfunctionayushnaithani004No ratings yet
- Male Reproductive SystemDocument26 pagesMale Reproductive SystemEmmanuelNo ratings yet
- Hormone Action in The Mammary Gland - C Brisken and B O Malley 2010Document15 pagesHormone Action in The Mammary Gland - C Brisken and B O Malley 2010tostonasNo ratings yet
- Bp503t Pcol Unit-VDocument46 pagesBp503t Pcol Unit-VAakkkNo ratings yet
- Tutorial 4.5Document4 pagesTutorial 4.5Rosa FinizioNo ratings yet
- Endocrine System 3 Adrenal Gland, Testes and OvariesDocument73 pagesEndocrine System 3 Adrenal Gland, Testes and OvariesdeeznutsnikkaballzNo ratings yet
- Endocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceFrom EverandEndocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceNo ratings yet
- Background of The ProjectDocument2 pagesBackground of The ProjectJolan Fernando HerceNo ratings yet
- Homework 1 Herce Bsmt2bDocument1 pageHomework 1 Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Biochemistry Herce Bsmt2bDocument1 pageBiochemistry Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Biochem Herce Bsmt2bDocument3 pagesBiochem Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Activity 1 Herce Bsmt1bDocument1 pageActivity 1 Herce Bsmt1bJolan Fernando HerceNo ratings yet
- Assessment QuestionnaireDocument3 pagesAssessment QuestionnaireJolan Fernando HerceNo ratings yet
- APznzab7n0tTt9B3o6nrEulLujxEm FmBRigdivDocument2 pagesAPznzab7n0tTt9B3o6nrEulLujxEm FmBRigdivJolan Fernando HerceNo ratings yet
- Activity On Endocrinology HERCE BSMT1BDocument1 pageActivity On Endocrinology HERCE BSMT1BJolan Fernando HerceNo ratings yet
- 20 Amino Acids Herce Bsmt2bDocument2 pages20 Amino Acids Herce Bsmt2bJolan Fernando HerceNo ratings yet
- Letter of Two Students For InterviewDocument1 pageLetter of Two Students For InterviewJolan Fernando HerceNo ratings yet
- Cytogen FnalsDocument17 pagesCytogen FnalsJolan Fernando HerceNo ratings yet
- SEATWORK1Document2 pagesSEATWORK1Jolan Fernando HerceNo ratings yet
- ACFrOgBSxI6Havvmnh9Te1qpS qBO9uYA HesicDocument6 pagesACFrOgBSxI6Havvmnh9Te1qpS qBO9uYA HesicJolan Fernando HerceNo ratings yet
- Covid19 Pandemic - HerceDocument6 pagesCovid19 Pandemic - HerceJolan Fernando HerceNo ratings yet
- Practical Test-Balancing and Types of Chemical RXNDocument1 pagePractical Test-Balancing and Types of Chemical RXNJolan Fernando HerceNo ratings yet
- Group Learning ActivityDocument3 pagesGroup Learning ActivityJolan Fernando HerceNo ratings yet
- Script For StepsDocument5 pagesScript For StepsJolan Fernando HerceNo ratings yet
- Medical Quackery and Folk MedicinesDocument2 pagesMedical Quackery and Folk MedicinesJolan Fernando HerceNo ratings yet
- Steps in Foodborne OutbreakDocument2 pagesSteps in Foodborne OutbreakJolan Fernando HerceNo ratings yet
- Biostat and Epi Transes SophomoresDocument60 pagesBiostat and Epi Transes SophomoresJolan Fernando HerceNo ratings yet
- Activity 8 - HERCE - BSMT1BDocument3 pagesActivity 8 - HERCE - BSMT1BJolan Fernando HerceNo ratings yet
- Activity Lab Sheet 4Document5 pagesActivity Lab Sheet 4Jolan Fernando HerceNo ratings yet
- Cytogen ReviewerDocument6 pagesCytogen ReviewerJolan Fernando HerceNo ratings yet
- QajjquoisiDocument41 pagesQajjquoisiJolan Fernando HerceNo ratings yet
- Mammo Prelim ReviewerDocument17 pagesMammo Prelim ReviewerCherie lou PizaNo ratings yet
- Stomac Și Duoden - LawrenceDocument45 pagesStomac Și Duoden - LawrenceNicole IoanidNo ratings yet
- Regulation of Cardiac Output, Intrinsic and Extrinsic FactorsDocument49 pagesRegulation of Cardiac Output, Intrinsic and Extrinsic FactorsPeter Mukunza100% (1)
- NHMRC Research Achievements - SUMMARY: Funded Research Into Injury Related Research ENDING 2004 TO 2013Document521 pagesNHMRC Research Achievements - SUMMARY: Funded Research Into Injury Related Research ENDING 2004 TO 2013Titoun LettounNo ratings yet
- Tera P90Document20 pagesTera P90samuel2koffiNo ratings yet
- Abdomen OSPE Charts Questions OnlyDocument29 pagesAbdomen OSPE Charts Questions OnlyRathnaNo ratings yet
- MER TopicsDocument4 pagesMER TopicsYaminiNo ratings yet
- Glaukoma Sekunder Pada Pasien Anak Dengan Sindrom Sturge Weber: Laporan KasusDocument7 pagesGlaukoma Sekunder Pada Pasien Anak Dengan Sindrom Sturge Weber: Laporan KasusLordisse WongNo ratings yet
- Tong Ren Poster - Janice GoldmanDocument1 pageTong Ren Poster - Janice Goldmanphani kumarNo ratings yet
- 組 員: 賴 冠 穎 U 1 0 7 S 1 4 6 游 育 瓏 U 1 0 8 0 2 0 1 洪 旻 謙 U 1 0 8 0 2 1 0 楊 易 達 U 1 0 8 0 2 5 9 簡 佳 瑋 U 1 0 7 0 1 6 4 謝 文 予 U 1 0 7 0 1 3 9Document13 pages組 員: 賴 冠 穎 U 1 0 7 S 1 4 6 游 育 瓏 U 1 0 8 0 2 0 1 洪 旻 謙 U 1 0 8 0 2 1 0 楊 易 達 U 1 0 8 0 2 5 9 簡 佳 瑋 U 1 0 7 0 1 6 4 謝 文 予 U 1 0 7 0 1 3 9William ChienNo ratings yet
- Human Memory 2nd Radvansky Test BankDocument9 pagesHuman Memory 2nd Radvansky Test BankJoshua Warren100% (38)
- Q4 - Module 3 Consumer ChemistryDocument23 pagesQ4 - Module 3 Consumer Chemistryjessie100% (2)
- Surgical Notes (Teddy)Document88 pagesSurgical Notes (Teddy)shichianNo ratings yet
- Self-Resolving Ischemic Third Nerve PalsyDocument10 pagesSelf-Resolving Ischemic Third Nerve PalsyYobbi ArisaputraNo ratings yet
- The Developing BrainDocument11 pagesThe Developing BrainRhodaNo ratings yet
- NCM 112 - Cardio 2 - Advance StudyDocument2 pagesNCM 112 - Cardio 2 - Advance StudyUzumaki KNo ratings yet
- Endocrine System: MSN 4006 Advanced PsychopathophysiologyDocument15 pagesEndocrine System: MSN 4006 Advanced Psychopathophysiologybane1925No ratings yet
- The Nervous System: EssentialsDocument27 pagesThe Nervous System: EssentialsSunshine LabraNo ratings yet
- Biomedicine & Pharmacotherapy: Original ArticleDocument8 pagesBiomedicine & Pharmacotherapy: Original ArticleYasmeen AbassNo ratings yet
- ReproductionDocument95 pagesReproductionSalman KhanNo ratings yet
- Seizure: Dr. Muhammad Yusuf, SP.S, FINSDocument24 pagesSeizure: Dr. Muhammad Yusuf, SP.S, FINSGina SoniaNo ratings yet
- Neurological DisordersDocument11 pagesNeurological DisordersFatima AsimNo ratings yet
- Hsslive Xi Zoology Rev Test 6 QN by Zta Malappuram - 230212 - 110004 PDFDocument4 pagesHsslive Xi Zoology Rev Test 6 QN by Zta Malappuram - 230212 - 110004 PDFഎസ്സാർപി ഊരുചുറ്റൽ തുടരുന്നുNo ratings yet
- Radiographic Features of RL LesionsDocument7 pagesRadiographic Features of RL Lesionszyad ahmedNo ratings yet
- 1 Klöppel Vortrag GEP NET The NE System 120217Document32 pages1 Klöppel Vortrag GEP NET The NE System 120217soledad88No ratings yet
- Gas 053Document11 pagesGas 053Angham OuadahNo ratings yet
- Small Animal Neurological Emergencies PLATTDocument673 pagesSmall Animal Neurological Emergencies PLATTrebeca garay100% (2)
- FTEC LESSON 34 Meat TechnologyDocument30 pagesFTEC LESSON 34 Meat Technologyjennelyn.antayNo ratings yet
- Burn Mmii - MMSS PDFDocument24 pagesBurn Mmii - MMSS PDFLuis A De la CubaNo ratings yet