Professional Documents
Culture Documents
VSEPRreferencechart 1
VSEPRreferencechart 1
Uploaded by
anjana ghelani0 ratings0% found this document useful (0 votes)
3 views1 pageSCH4U U2 VESPR Chart
Original Title
VSEPRreferencechart-1
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSCH4U U2 VESPR Chart
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
3 views1 pageVSEPRreferencechart 1
VSEPRreferencechart 1
Uploaded by
anjana ghelaniSCH4U U2 VESPR Chart
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 1
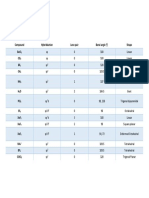
Central atom
Lone Shape Angle Hybridization Example Lewis structure
Bonds
pairs
2 0 Linear 180 sp CO2 16e-
1 1 Linear n/a sp CN- 10e-
3 0 Trigonal planar 120 sp2 NO3-1 24e-
2 1 Bent 118 sp2 BH2-1 6e-
1 2 Linear n/a sp2 O2 12e-
4 0 Tetrahedral 109.5 sp3 CH4 8e-
3 1 Trigonal Pyramidal 107.5 sp3 NH3 8e-
2 2 Bent 104.5 sp3 H2O 8e-
1 3 Linear n/a sp3 OH- 8e-
5 0 Trigonal bipyramidal 120, 90 sp3d PF5 40e-
4 1 See Saw 118, <90 sp3d PF4- 34e-
Trigonal planar or T-
3 2 120 sp3d PF3-2 28e-
shaped
2 3 Linear 180 sp3d I3-1 22e-
1 4 Linear n/a sp3d S2-4 16e-
6 0 Octahedral 90 sp3d2 SF6 48e-
5 1 Square pyramidal <90 sp3d2 IF5 42e-
4 2 Square planar 90 sp3d2 XeF4 36e-
3 3 T-shaped <90 sp3d2 XeF3-1 30e-
2 4 Linear 180 sp3d2 XeF2-2 24e-
1 5 Linear n/a sp3d2 XeF-3 18e-
You might also like
- Lewis Structures and Molecular GeometryDocument2 pagesLewis Structures and Molecular GeometryrsleoNo ratings yet
- Compound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclDocument1 pageCompound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclSakib KhanNo ratings yet
- VSEPR and Molecular Geometries (Summery)Document2 pagesVSEPR and Molecular Geometries (Summery)MihadNo ratings yet
- Q5 GradedDocument5 pagesQ5 GradedParam Veer ChoudharyNo ratings yet
- RT46K6A4KS9Document8 pagesRT46K6A4KS9Tom MartinsNo ratings yet
- Materials Comparison DIN / EN / ASTM: Finished PartsDocument6 pagesMaterials Comparison DIN / EN / ASTM: Finished PartsBittuNo ratings yet
- Adobe Scan Jan 08, 2022Document1 pageAdobe Scan Jan 08, 2022Jony MohammedNo ratings yet
- Needle TypesDocument2 pagesNeedle TypesKarishmaNo ratings yet
- Dacs Class Work 10augDocument4 pagesDacs Class Work 10augBharath SNo ratings yet
- Steric No. Form Shape Angle HybridizationDocument1 pageSteric No. Form Shape Angle Hybridizationmica_tsukadaNo ratings yet
- Ecuacion de FlujoDocument2 pagesEcuacion de FlujoRamiro BritoNo ratings yet
- Alfabeto RectaDocument1 pageAlfabeto RectaALVARO CARMENA CARMENANo ratings yet
- Blowers: Blower Motors, Wheels & FansDocument27 pagesBlowers: Blower Motors, Wheels & FansAndrey GyrychNo ratings yet
- Materials Comparison DIN / EN / ASTM: Pipes / Tubes Flanges Buttwelding FittingsDocument1 pageMaterials Comparison DIN / EN / ASTM: Pipes / Tubes Flanges Buttwelding FittingsMiguel MachadoNo ratings yet
- Material ComparisonDocument1 pageMaterial ComparisonSetiyo Bayu IndrayanaNo ratings yet
- Class: Xi Inorganic Chemistry DPP. NO.-9Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-9Radhika MohataNo ratings yet
- Chinese Pixie - 3rd Planet Solar - KC9ON Filtro de Salida Otras Bandas 2Document2 pagesChinese Pixie - 3rd Planet Solar - KC9ON Filtro de Salida Otras Bandas 2Diego García MedinaNo ratings yet
- ESci110 Pre-Cal - Uriarte - BSABE1 - Lesson 11.3 AssessmentDocument4 pagesESci110 Pre-Cal - Uriarte - BSABE1 - Lesson 11.3 AssessmentMa. Derlina MoraNo ratings yet
- Molecular ModelDocument1 pageMolecular ModelPedro SuyuNo ratings yet
- Tablica Vzaimozamenamosti Torcevie UplotneniaDocument1 pageTablica Vzaimozamenamosti Torcevie UplotneniaАйрат ГараевNo ratings yet
- Tarea 7. Síntesis y Diseño Detallado A Partir de Modelización de Sist. ContinuosDocument5 pagesTarea 7. Síntesis y Diseño Detallado A Partir de Modelización de Sist. Continuosdanielalejandro1022No ratings yet
- COMPARISON OF STEEL GRADES - Part7Document1 pageCOMPARISON OF STEEL GRADES - Part7Ujwal KhandokarNo ratings yet
- VRATILO Petar FinishDocument28 pagesVRATILO Petar FinishPetar JuricNo ratings yet
- Tonar Katalog 22-31sDocument10 pagesTonar Katalog 22-31schampionNo ratings yet
- Monofazni MotoriDocument12 pagesMonofazni MotoriNandor Kermeci100% (1)
- NB 176e General Catalog v2Document315 pagesNB 176e General Catalog v2dudungprerNo ratings yet
- List of Uniform Polyhedra: From Wikipedia, The Free EncyclopediaDocument13 pagesList of Uniform Polyhedra: From Wikipedia, The Free EncyclopediaIIRemmyIINo ratings yet
- F - KD - Peng - SBDP (KELAS 6)Document10 pagesF - KD - Peng - SBDP (KELAS 6)Husniaty HusniatyNo ratings yet
- RM Semana 11Document18 pagesRM Semana 11eduardo rodriguezNo ratings yet
- Thời Khóa Biểu Tkb-Hki Năm Học 2018-2019 Áp Dụng Ngày 22/4/2019Document3 pagesThời Khóa Biểu Tkb-Hki Năm Học 2018-2019 Áp Dụng Ngày 22/4/2019Nguyễn SangNo ratings yet
- Kamal - New Plant ToolsDocument6 pagesKamal - New Plant Toolsranj kNo ratings yet
- CHM 201 2019-2020 Note1Document38 pagesCHM 201 2019-2020 Note1Adams TemitopeNo ratings yet
- 2.60 SP Series-MinDocument23 pages2.60 SP Series-MinKikuchi ToumaNo ratings yet
- F KD Ket Prakarya 8ADocument4 pagesF KD Ket Prakarya 8AAbdul Wahid HaqiqiNo ratings yet
- Thyristor TRIAC Qxx40xx A SPICE Model LibDocument8 pagesThyristor TRIAC Qxx40xx A SPICE Model LibJosé G.No ratings yet
- List Bolt, Gasket, Valve Size U-337Document70 pagesList Bolt, Gasket, Valve Size U-337daniNo ratings yet
- Vsepr HandoutDocument2 pagesVsepr HandoutAdrianne Jericho ValdezNo ratings yet
- VSEPR Handout PDFDocument2 pagesVSEPR Handout PDFLittle One0% (1)
- Vsepr HandoutDocument2 pagesVsepr Handout20718 LAY BUFFON FERNANDO GROSSONo ratings yet
- SFB 2 Series Relay ManualDocument5 pagesSFB 2 Series Relay ManualKunjan DalwadiNo ratings yet
- Obtan Stat Ep: 12tkejted EDocument6 pagesObtan Stat Ep: 12tkejted EViha NaikNo ratings yet
- Question 3rdDocument18 pagesQuestion 3rdkaranNo ratings yet
- Rt38jhrbdsl CL 01Document8 pagesRt38jhrbdsl CL 01Lendys DnielNo ratings yet
- ARML Local 2021 AnswersDocument1 pageARML Local 2021 AnswersQun LiNo ratings yet
- Kody SMD cz.2Document83 pagesKody SMD cz.2Dariusz DARCIONo ratings yet
- SMRT Refurb DrawingDocument41 pagesSMRT Refurb DrawingFarhan RahmanNo ratings yet
- Uht 75 Uht 79Document1 pageUht 75 Uht 79ALI MESSAOUDINo ratings yet
- Reloop SMDocument36 pagesReloop SMAsad AhmedNo ratings yet
- 7200-5.mpa 133-24 4 KN - T: 159.93 EDocument2 pages7200-5.mpa 133-24 4 KN - T: 159.93 EJEHRAM DYL NAVALESNo ratings yet
- F - KD - Ket - Prakarya - IX ADocument4 pagesF - KD - Ket - Prakarya - IX Asofwan akhmadNo ratings yet
- AWS Classifications of ElectrodesDocument2 pagesAWS Classifications of ElectrodesJorge SobrevillaNo ratings yet
- Commercial Unit CoolersDocument2 pagesCommercial Unit CoolersClarence JarlosNo ratings yet
- En5509 KH3P-KH4PDocument4 pagesEn5509 KH3P-KH4PCarlos GraterolNo ratings yet
- R 1028 50 R 3 - 978 50 T TDocument8 pagesR 1028 50 R 3 - 978 50 T TJorgeCarlosNo ratings yet
- F - Ketermpl - MTK (Kelas 1)Document4 pagesF - Ketermpl - MTK (Kelas 1)Desa SukosariNo ratings yet
- F - KD - Peng - Prakarya - IX ADocument4 pagesF - KD - Peng - Prakarya - IX Asofwan akhmadNo ratings yet
- 3pc de FQ MonteroDocument5 pages3pc de FQ MonteroKEVIN ANDREE MONTERO FERNANDEZNo ratings yet
- Section: New NewDocument32 pagesSection: New NewlaquanNo ratings yet
- Copeland Scroll ZRK5 CompressorDocument16 pagesCopeland Scroll ZRK5 Compressorrlynch33No ratings yet