Professional Documents
Culture Documents
2A Checklist
2A Checklist
Uploaded by
starqumiCopyright:
Available Formats
You might also like
- Full Download Book Breast Mri State of The Art and Future Directions PDFDocument41 pagesFull Download Book Breast Mri State of The Art and Future Directions PDFedith.mays17897% (30)
- Nursery To Class 5 NOF Junior International Olympiads - Examination ScheduleDocument18 pagesNursery To Class 5 NOF Junior International Olympiads - Examination ScheduleRenu TyagiNo ratings yet
- Aircraft Maintenance Engineering: Nanyang Technological UniversityDocument31 pagesAircraft Maintenance Engineering: Nanyang Technological UniversitySaitama FishNo ratings yet
- Chemistry PAG 2.1 Learner v2.3 2 3Document3 pagesChemistry PAG 2.1 Learner v2.3 2 33t4e5yuezryhNo ratings yet
- Chemistry 1 Practical and Lab Report-Sodium CarbonateDocument3 pagesChemistry 1 Practical and Lab Report-Sodium CarbonateAbdelrahman MahmoudNo ratings yet
- Experiment 2 Standardization of Sodium Hydroxide With HCLDocument2 pagesExperiment 2 Standardization of Sodium Hydroxide With HCLvafaashkNo ratings yet
- Practical Handbook OnDocument47 pagesPractical Handbook OnSleepyHead ˋωˊ100% (1)
- 韩篙夫 62017010079 Assignment2Document5 pages韩篙夫 62017010079 Assignment2Hasan Galiv NabinNo ratings yet
- Sample Learner Work - No ColDocument15 pagesSample Learner Work - No ColShabaz SaysNo ratings yet
- Acid-Base Titration1 PDFDocument28 pagesAcid-Base Titration1 PDFMarc DanielNo ratings yet
- PracticalHandbookon PDFDocument48 pagesPracticalHandbookon PDFderplNo ratings yet
- Introduction To EURACHEM/CITAC Uncertainty Guide 2 EditionDocument30 pagesIntroduction To EURACHEM/CITAC Uncertainty Guide 2 EditionSairam KishoreNo ratings yet
- White Paper Measurement of Uncertainty in TitrationDocument12 pagesWhite Paper Measurement of Uncertainty in TitrationAbdulazeez Omer AlmadehNo ratings yet
- Draft Oecd Guideline For The Testing of ChemicalsDocument7 pagesDraft Oecd Guideline For The Testing of ChemicalsMauricio SilvestriNo ratings yet
- PH, TA& % AcidityDocument41 pagesPH, TA& % Acidityasutoshpanda618No ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- Acid-Base Titrations and PH DeterminationDocument3 pagesAcid-Base Titrations and PH DeterminationDan DomagalaNo ratings yet
- CalciumDocument4 pagesCalciumrancho13No ratings yet
- DownloadDocument5 pagesDownloadeseperrorrosinsinNo ratings yet
- Lab 15 PD Qualitative AnalysisDocument5 pagesLab 15 PD Qualitative AnalysisAnahi BeltranNo ratings yet
- Acid Base Titrations LabDocument3 pagesAcid Base Titrations LabLoveena Steadman100% (1)
- Biuret MethodDocument2 pagesBiuret MethodLarry LucianoNo ratings yet
- Introduction of Pharmaceutical Analytical PharmacyDocument28 pagesIntroduction of Pharmaceutical Analytical PharmacyCalvin HansNo ratings yet
- 04 (2) HJBHDocument3 pages04 (2) HJBHncubepharmaNo ratings yet
- Diclofenac Rabeprazole HPLCDocument5 pagesDiclofenac Rabeprazole HPLCdeepscpn1571No ratings yet
- 62 Experiment #5. Titration of An Acid Using A PH MeterDocument7 pages62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemNo ratings yet
- Sem 2Document58 pagesSem 2ZavashNo ratings yet
- Practice Nº1 Acid Base VolumetryDocument6 pagesPractice Nº1 Acid Base VolumetryScribdTranslationsNo ratings yet
- Guide To Practical WorkDocument96 pagesGuide To Practical WorkDivya BajpaiNo ratings yet
- 103-3IS Des Ch5 Formative Crit B & C Test (YC4W1Y)Document8 pages103-3IS Des Ch5 Formative Crit B & C Test (YC4W1Y)Stephonia dsouzwNo ratings yet
- Set B Cluster 1 Final 081015Document6 pagesSet B Cluster 1 Final 081015Jose Marie AsuncionNo ratings yet
- CE8512-Water and Waste Water Analysis LaboratoryDocument97 pagesCE8512-Water and Waste Water Analysis LaboratoryVICTORYSUBIKSHINo ratings yet
- Estimation MUCl ASTMD512 Final 20220907 For RGRev 1Document29 pagesEstimation MUCl ASTMD512 Final 20220907 For RGRev 1Eco Environmental Consultancy ServicesNo ratings yet
- Analytical method validationDocument9 pagesAnalytical method validationJai MurugeshNo ratings yet
- The Evaluation of Measurement Uncertainty From Method Validation StudiesDocument10 pagesThe Evaluation of Measurement Uncertainty From Method Validation StudiesKhalidNo ratings yet
- Chemistry Lab Book EnglishDocument81 pagesChemistry Lab Book EnglishSmile ChoudharyNo ratings yet
- Chem TechDocument7 pagesChem TechJielyn Monreal CornelioNo ratings yet
- G7 Stomach Ache Criterion B and CDocument9 pagesG7 Stomach Ache Criterion B and CDark guyNo ratings yet
- Analytical Method Validation For Cleaning ValidationDocument14 pagesAnalytical Method Validation For Cleaning Validationcurezahealthcare111No ratings yet
- Postlab 1Document4 pagesPostlab 1Paul ColoyanNo ratings yet
- Analytical Method Validation Protocol For Pharmaceuticals - Pharmaceutical GuidelinesDocument7 pagesAnalytical Method Validation Protocol For Pharmaceuticals - Pharmaceutical GuidelinesMSL IndiaNo ratings yet
- Experiment of Determination of PH Solids and HardnessDocument40 pagesExperiment of Determination of PH Solids and HardnessNasrulNo ratings yet
- Practical Syllabus Grade 11Document4 pagesPractical Syllabus Grade 11arkdhokriyaNo ratings yet
- Lab Manual CHM510Document43 pagesLab Manual CHM510marzNo ratings yet
- Referencia PH 1990060001 - HC15456606 - SU - ENDocument2 pagesReferencia PH 1990060001 - HC15456606 - SU - ENicaro89No ratings yet
- Week 5.analytical ChemistryDocument43 pagesWeek 5.analytical ChemistryChi NguyenNo ratings yet
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- CHEM A 14A COMP Qual CationsDocument7 pagesCHEM A 14A COMP Qual CationscesarNo ratings yet
- Tirtha S HGAAS - Experiment Final (ARTIKAN)Document9 pagesTirtha S HGAAS - Experiment Final (ARTIKAN)Rizka MaharanaNo ratings yet
- Revised Lab Manual 14-12-2018Document299 pagesRevised Lab Manual 14-12-2018Abhishek NayakNo ratings yet
- Module4 - Volumetric Method of AnalysisDocument18 pagesModule4 - Volumetric Method of AnalysisJoyce Mariele RomeroNo ratings yet
- Chapter 7-Titrations (Taking Adv. of Stoich. Reactions)Document24 pagesChapter 7-Titrations (Taking Adv. of Stoich. Reactions)vada_soNo ratings yet
- Chapter 1 Rev 3Document36 pagesChapter 1 Rev 3Roger FernandezNo ratings yet
- Mole Ratios and Reaction StoichiometryDocument4 pagesMole Ratios and Reaction StoichiometryAishaNo ratings yet
- ABCT3631 Metrological TraceabilityDocument18 pagesABCT3631 Metrological Traceabilitykitkitaoa2002No ratings yet
- Activity 3 UpdatedDocument4 pagesActivity 3 UpdatedDraco PhoenixNo ratings yet
- Titration Guide MS - PG1682EN PDFDocument36 pagesTitration Guide MS - PG1682EN PDFCJ PrettyNo ratings yet
- Experiment 4: Flow Injection Analyst System (FIAS) 1.0Document4 pagesExperiment 4: Flow Injection Analyst System (FIAS) 1.0Nurul HanisNo ratings yet
- Quantitative Determination of Potassium Acid Phthalate KHPDocument17 pagesQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilNo ratings yet
- Experiment 1 - Formal Report - Aguilar Alih BassarDocument15 pagesExperiment 1 - Formal Report - Aguilar Alih Bassarmedz dharNo ratings yet
- BQC 1K051D 24022616Document1 pageBQC 1K051D 24022616josman733No ratings yet
- Chem Lab ManualDocument37 pagesChem Lab ManualChris JonathanNo ratings yet
- Recognition PDFDocument2 pagesRecognition PDFSDL Malkapur BuldanaNo ratings yet
- Transverse Disciplines in Metrology: Proceedings of the 13th International Metrology Congress, 2007 - Lille, FranceFrom EverandTransverse Disciplines in Metrology: Proceedings of the 13th International Metrology Congress, 2007 - Lille, FranceNo ratings yet
- ColorimetryDocument15 pagesColorimetrystarqumiNo ratings yet
- WP Contentuploads202212criminology Student WB - U1 PDFDocument104 pagesWP Contentuploads202212criminology Student WB - U1 PDFstarqumiNo ratings yet
- MTW Application Form NotesDocument1 pageMTW Application Form NotesstarqumiNo ratings yet
- 000895a-Hate-Crimes-2018-2020-1 CriminologyDocument187 pages000895a-Hate-Crimes-2018-2020-1 CriminologystarqumiNo ratings yet
- Year 11 GCSE Revision Cards 2023Document1 pageYear 11 GCSE Revision Cards 2023starqumiNo ratings yet
- A Technical Report On Industrial Training Done at NCAMDocument26 pagesA Technical Report On Industrial Training Done at NCAMRidwanullah AbdulrahmanNo ratings yet
- Mariano Marcos State University: College of Teacher Education Laoag City, Ilocos NorteDocument6 pagesMariano Marcos State University: College of Teacher Education Laoag City, Ilocos NorteEisle Keith Rivera TapiaNo ratings yet
- A) Preparation of The Standard Calcium SolutionsDocument2 pagesA) Preparation of The Standard Calcium Solutionshahaamin aminNo ratings yet
- KAHAN, D (2016) - Politically Motivated ReasoningDocument16 pagesKAHAN, D (2016) - Politically Motivated ReasoningRafaella RibeiroNo ratings yet
- BE Auto SyllabusDocument91 pagesBE Auto SyllabusMan mNo ratings yet
- A Study of Best Prctice of Schedulled Outage Program in Increase Plant PerformanceDocument28 pagesA Study of Best Prctice of Schedulled Outage Program in Increase Plant PerformanceYULIANTONo ratings yet
- Facts Versus OpinionDocument20 pagesFacts Versus OpinionIrish Ga-aNo ratings yet
- Gen - Math - Answersheet (Module 8)Document2 pagesGen - Math - Answersheet (Module 8)JERLYN MACADONo ratings yet
- Introducing Social Psychology: Total Assessment Guide (TAG)Document62 pagesIntroducing Social Psychology: Total Assessment Guide (TAG)Rubaiya ChowdhuryNo ratings yet
- Importance of Soil Study in Civil EngineeringDocument6 pagesImportance of Soil Study in Civil EngineeringJack FosterNo ratings yet
- Exercise - 1 To 3 STDocument11 pagesExercise - 1 To 3 STSky SirNo ratings yet
- Evolution of WarfareDocument8 pagesEvolution of WarfareMatt LoweNo ratings yet
- CC511 Week 5 - 6 - NN - BPDocument62 pagesCC511 Week 5 - 6 - NN - BPmohamed sherifNo ratings yet
- Esas Module (Concepts) : Encoded By: Fausto, Dimazana, Matuto, Maniquis, Montanno, MalicdemDocument313 pagesEsas Module (Concepts) : Encoded By: Fausto, Dimazana, Matuto, Maniquis, Montanno, MalicdemAlven PullaNo ratings yet
- Detailed Lesson Plan in Science Grade 6Document7 pagesDetailed Lesson Plan in Science Grade 6Mary Joy RobisNo ratings yet
- Mr. Clutter Villegas Middle SchoolDocument9 pagesMr. Clutter Villegas Middle Schoolgilbert_espinosaNo ratings yet
- NSTP CTRL FDocument29 pagesNSTP CTRL FCherie QuintoNo ratings yet
- Cooling Tower Alignment - LaserDocument3 pagesCooling Tower Alignment - LasersyamsidiNo ratings yet
- Pentens Industrial Flooring Solution NewDocument32 pagesPentens Industrial Flooring Solution Newウィリアムズ アンディNo ratings yet
- Fire Is The Rapid Oxidation of A Material in The ExothermicDocument4 pagesFire Is The Rapid Oxidation of A Material in The ExothermicIllavarasanNo ratings yet
- Volume+2 +Table+of+Contents+&+Chapter+17+The+ShiftsDocument36 pagesVolume+2 +Table+of+Contents+&+Chapter+17+The+ShiftsRehan BaigNo ratings yet
- Graham Holton - UFOs and Religious CultsDocument23 pagesGraham Holton - UFOs and Religious CultsJorsant EdNo ratings yet
- Rsos 171773Document17 pagesRsos 171773JULIO CÉSAR CHÁVEZ GALARZANo ratings yet
- 2 Lesson Notes Instrument DatasheetDocument5 pages2 Lesson Notes Instrument Datasheetmubanga20000804100% (1)
- How To Perform Surgical Hand ScrubsDocument4 pagesHow To Perform Surgical Hand ScrubsInna XiiNo ratings yet
- Attachment 1-YICMG2024 Competition ThemeDocument5 pagesAttachment 1-YICMG2024 Competition Themevulananh838No ratings yet
2A Checklist
2A Checklist
Uploaded by
starqumiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2A Checklist
2A Checklist
Uploaded by
starqumiCopyright:
Available Formats
Unit 2 Assignment A: Concentrate of keeping up your standards
Checklist
Assessment Task to Complete Done?
Criteria
A.P1 Titration: Calibrate pipette, weighing balance (analytical 4dp and rough balance
2dp with certified weights) and the pH meter (using manufacturing instructions
and buffer solutions)
Colorimetry: Calibrate the Colorimeterq
Risk assessments for:
a) Making a standard solution of Sodium Carbonate (weighing solids
accurately)
b) Standardisation of an acid
c) Titration of Sodium Hydroxide with Hydrochloric Acid – using an indicator
and using a pH meter
d) Determining the concentration of Copper (ii) Sulfate solution by
colorimetry
Method and equipment list for making a standard solution of Sodium Carbonate
Method and equipment list for standardisation of an acid
Method and equipment list for titration of sodium hydroxide with hydrochloric acid
with:
a) Indicator
b) pH meter
Method and equipment list for determining the concentration of copper (II) sulfate
by colorimetry
Prepare Sodium Carbonate standard solution
Standardisation of an acid practical
A.P2 Conduct the titration of Sodium Hydroxide solution with the standardised
hydrochloric acid using an indicator (plus repeats). Result tables
Conduct the titration of Sodium Hydroxide solution with the standardised
hydrochloric acid using a calibrated pH meter (plus repeats). Result tables
Investigate the concentrate of Copper (II) Sulfate solution by colorimetry. Result
tables
A.M1 Fully reference any sources of information
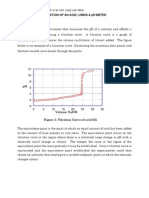
Calculate concentrations from titration readings and from a graph of pH against
volume of HCl
Describe the differences between primary and secondary titrimetric standards
Select an appropriate filter/wavelength for the colorimetry
Use the calibration curve to determine the concentrations of sample A and B
A.D1 Evaluate the accuracy of your results, providing sound justification and reasoning
and comparing the concentrations you have determined with actual
concentrations (publishers) and those of others.
Explain how accuracy, precision and reliability were ensured during your
experiments, and discuss any problems or issues that occurred during
performance of your experiments
Propose improvements or alternatives to the procedures and techniques,
providing a strong rationale why they will improve accuracy, precision, reliability or
validity
Review your risk assessment and justify the way in which certain procedures
were carried out in a particular way on grounds of safety.
You might also like
- Full Download Book Breast Mri State of The Art and Future Directions PDFDocument41 pagesFull Download Book Breast Mri State of The Art and Future Directions PDFedith.mays17897% (30)
- Nursery To Class 5 NOF Junior International Olympiads - Examination ScheduleDocument18 pagesNursery To Class 5 NOF Junior International Olympiads - Examination ScheduleRenu TyagiNo ratings yet
- Aircraft Maintenance Engineering: Nanyang Technological UniversityDocument31 pagesAircraft Maintenance Engineering: Nanyang Technological UniversitySaitama FishNo ratings yet
- Chemistry PAG 2.1 Learner v2.3 2 3Document3 pagesChemistry PAG 2.1 Learner v2.3 2 33t4e5yuezryhNo ratings yet
- Chemistry 1 Practical and Lab Report-Sodium CarbonateDocument3 pagesChemistry 1 Practical and Lab Report-Sodium CarbonateAbdelrahman MahmoudNo ratings yet
- Experiment 2 Standardization of Sodium Hydroxide With HCLDocument2 pagesExperiment 2 Standardization of Sodium Hydroxide With HCLvafaashkNo ratings yet
- Practical Handbook OnDocument47 pagesPractical Handbook OnSleepyHead ˋωˊ100% (1)
- 韩篙夫 62017010079 Assignment2Document5 pages韩篙夫 62017010079 Assignment2Hasan Galiv NabinNo ratings yet
- Sample Learner Work - No ColDocument15 pagesSample Learner Work - No ColShabaz SaysNo ratings yet
- Acid-Base Titration1 PDFDocument28 pagesAcid-Base Titration1 PDFMarc DanielNo ratings yet
- PracticalHandbookon PDFDocument48 pagesPracticalHandbookon PDFderplNo ratings yet
- Introduction To EURACHEM/CITAC Uncertainty Guide 2 EditionDocument30 pagesIntroduction To EURACHEM/CITAC Uncertainty Guide 2 EditionSairam KishoreNo ratings yet
- White Paper Measurement of Uncertainty in TitrationDocument12 pagesWhite Paper Measurement of Uncertainty in TitrationAbdulazeez Omer AlmadehNo ratings yet
- Draft Oecd Guideline For The Testing of ChemicalsDocument7 pagesDraft Oecd Guideline For The Testing of ChemicalsMauricio SilvestriNo ratings yet
- PH, TA& % AcidityDocument41 pagesPH, TA& % Acidityasutoshpanda618No ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- Acid-Base Titrations and PH DeterminationDocument3 pagesAcid-Base Titrations and PH DeterminationDan DomagalaNo ratings yet
- CalciumDocument4 pagesCalciumrancho13No ratings yet
- DownloadDocument5 pagesDownloadeseperrorrosinsinNo ratings yet
- Lab 15 PD Qualitative AnalysisDocument5 pagesLab 15 PD Qualitative AnalysisAnahi BeltranNo ratings yet
- Acid Base Titrations LabDocument3 pagesAcid Base Titrations LabLoveena Steadman100% (1)
- Biuret MethodDocument2 pagesBiuret MethodLarry LucianoNo ratings yet
- Introduction of Pharmaceutical Analytical PharmacyDocument28 pagesIntroduction of Pharmaceutical Analytical PharmacyCalvin HansNo ratings yet
- 04 (2) HJBHDocument3 pages04 (2) HJBHncubepharmaNo ratings yet
- Diclofenac Rabeprazole HPLCDocument5 pagesDiclofenac Rabeprazole HPLCdeepscpn1571No ratings yet
- 62 Experiment #5. Titration of An Acid Using A PH MeterDocument7 pages62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemNo ratings yet
- Sem 2Document58 pagesSem 2ZavashNo ratings yet
- Practice Nº1 Acid Base VolumetryDocument6 pagesPractice Nº1 Acid Base VolumetryScribdTranslationsNo ratings yet
- Guide To Practical WorkDocument96 pagesGuide To Practical WorkDivya BajpaiNo ratings yet
- 103-3IS Des Ch5 Formative Crit B & C Test (YC4W1Y)Document8 pages103-3IS Des Ch5 Formative Crit B & C Test (YC4W1Y)Stephonia dsouzwNo ratings yet
- Set B Cluster 1 Final 081015Document6 pagesSet B Cluster 1 Final 081015Jose Marie AsuncionNo ratings yet
- CE8512-Water and Waste Water Analysis LaboratoryDocument97 pagesCE8512-Water and Waste Water Analysis LaboratoryVICTORYSUBIKSHINo ratings yet
- Estimation MUCl ASTMD512 Final 20220907 For RGRev 1Document29 pagesEstimation MUCl ASTMD512 Final 20220907 For RGRev 1Eco Environmental Consultancy ServicesNo ratings yet
- Analytical method validationDocument9 pagesAnalytical method validationJai MurugeshNo ratings yet
- The Evaluation of Measurement Uncertainty From Method Validation StudiesDocument10 pagesThe Evaluation of Measurement Uncertainty From Method Validation StudiesKhalidNo ratings yet
- Chemistry Lab Book EnglishDocument81 pagesChemistry Lab Book EnglishSmile ChoudharyNo ratings yet
- Chem TechDocument7 pagesChem TechJielyn Monreal CornelioNo ratings yet
- G7 Stomach Ache Criterion B and CDocument9 pagesG7 Stomach Ache Criterion B and CDark guyNo ratings yet
- Analytical Method Validation For Cleaning ValidationDocument14 pagesAnalytical Method Validation For Cleaning Validationcurezahealthcare111No ratings yet
- Postlab 1Document4 pagesPostlab 1Paul ColoyanNo ratings yet
- Analytical Method Validation Protocol For Pharmaceuticals - Pharmaceutical GuidelinesDocument7 pagesAnalytical Method Validation Protocol For Pharmaceuticals - Pharmaceutical GuidelinesMSL IndiaNo ratings yet
- Experiment of Determination of PH Solids and HardnessDocument40 pagesExperiment of Determination of PH Solids and HardnessNasrulNo ratings yet
- Practical Syllabus Grade 11Document4 pagesPractical Syllabus Grade 11arkdhokriyaNo ratings yet
- Lab Manual CHM510Document43 pagesLab Manual CHM510marzNo ratings yet
- Referencia PH 1990060001 - HC15456606 - SU - ENDocument2 pagesReferencia PH 1990060001 - HC15456606 - SU - ENicaro89No ratings yet
- Week 5.analytical ChemistryDocument43 pagesWeek 5.analytical ChemistryChi NguyenNo ratings yet
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- CHEM A 14A COMP Qual CationsDocument7 pagesCHEM A 14A COMP Qual CationscesarNo ratings yet
- Tirtha S HGAAS - Experiment Final (ARTIKAN)Document9 pagesTirtha S HGAAS - Experiment Final (ARTIKAN)Rizka MaharanaNo ratings yet
- Revised Lab Manual 14-12-2018Document299 pagesRevised Lab Manual 14-12-2018Abhishek NayakNo ratings yet
- Module4 - Volumetric Method of AnalysisDocument18 pagesModule4 - Volumetric Method of AnalysisJoyce Mariele RomeroNo ratings yet
- Chapter 7-Titrations (Taking Adv. of Stoich. Reactions)Document24 pagesChapter 7-Titrations (Taking Adv. of Stoich. Reactions)vada_soNo ratings yet
- Chapter 1 Rev 3Document36 pagesChapter 1 Rev 3Roger FernandezNo ratings yet
- Mole Ratios and Reaction StoichiometryDocument4 pagesMole Ratios and Reaction StoichiometryAishaNo ratings yet
- ABCT3631 Metrological TraceabilityDocument18 pagesABCT3631 Metrological Traceabilitykitkitaoa2002No ratings yet
- Activity 3 UpdatedDocument4 pagesActivity 3 UpdatedDraco PhoenixNo ratings yet
- Titration Guide MS - PG1682EN PDFDocument36 pagesTitration Guide MS - PG1682EN PDFCJ PrettyNo ratings yet
- Experiment 4: Flow Injection Analyst System (FIAS) 1.0Document4 pagesExperiment 4: Flow Injection Analyst System (FIAS) 1.0Nurul HanisNo ratings yet
- Quantitative Determination of Potassium Acid Phthalate KHPDocument17 pagesQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilNo ratings yet
- Experiment 1 - Formal Report - Aguilar Alih BassarDocument15 pagesExperiment 1 - Formal Report - Aguilar Alih Bassarmedz dharNo ratings yet
- BQC 1K051D 24022616Document1 pageBQC 1K051D 24022616josman733No ratings yet
- Chem Lab ManualDocument37 pagesChem Lab ManualChris JonathanNo ratings yet
- Recognition PDFDocument2 pagesRecognition PDFSDL Malkapur BuldanaNo ratings yet
- Transverse Disciplines in Metrology: Proceedings of the 13th International Metrology Congress, 2007 - Lille, FranceFrom EverandTransverse Disciplines in Metrology: Proceedings of the 13th International Metrology Congress, 2007 - Lille, FranceNo ratings yet
- ColorimetryDocument15 pagesColorimetrystarqumiNo ratings yet
- WP Contentuploads202212criminology Student WB - U1 PDFDocument104 pagesWP Contentuploads202212criminology Student WB - U1 PDFstarqumiNo ratings yet
- MTW Application Form NotesDocument1 pageMTW Application Form NotesstarqumiNo ratings yet
- 000895a-Hate-Crimes-2018-2020-1 CriminologyDocument187 pages000895a-Hate-Crimes-2018-2020-1 CriminologystarqumiNo ratings yet
- Year 11 GCSE Revision Cards 2023Document1 pageYear 11 GCSE Revision Cards 2023starqumiNo ratings yet
- A Technical Report On Industrial Training Done at NCAMDocument26 pagesA Technical Report On Industrial Training Done at NCAMRidwanullah AbdulrahmanNo ratings yet
- Mariano Marcos State University: College of Teacher Education Laoag City, Ilocos NorteDocument6 pagesMariano Marcos State University: College of Teacher Education Laoag City, Ilocos NorteEisle Keith Rivera TapiaNo ratings yet
- A) Preparation of The Standard Calcium SolutionsDocument2 pagesA) Preparation of The Standard Calcium Solutionshahaamin aminNo ratings yet
- KAHAN, D (2016) - Politically Motivated ReasoningDocument16 pagesKAHAN, D (2016) - Politically Motivated ReasoningRafaella RibeiroNo ratings yet
- BE Auto SyllabusDocument91 pagesBE Auto SyllabusMan mNo ratings yet
- A Study of Best Prctice of Schedulled Outage Program in Increase Plant PerformanceDocument28 pagesA Study of Best Prctice of Schedulled Outage Program in Increase Plant PerformanceYULIANTONo ratings yet
- Facts Versus OpinionDocument20 pagesFacts Versus OpinionIrish Ga-aNo ratings yet
- Gen - Math - Answersheet (Module 8)Document2 pagesGen - Math - Answersheet (Module 8)JERLYN MACADONo ratings yet
- Introducing Social Psychology: Total Assessment Guide (TAG)Document62 pagesIntroducing Social Psychology: Total Assessment Guide (TAG)Rubaiya ChowdhuryNo ratings yet
- Importance of Soil Study in Civil EngineeringDocument6 pagesImportance of Soil Study in Civil EngineeringJack FosterNo ratings yet
- Exercise - 1 To 3 STDocument11 pagesExercise - 1 To 3 STSky SirNo ratings yet
- Evolution of WarfareDocument8 pagesEvolution of WarfareMatt LoweNo ratings yet
- CC511 Week 5 - 6 - NN - BPDocument62 pagesCC511 Week 5 - 6 - NN - BPmohamed sherifNo ratings yet
- Esas Module (Concepts) : Encoded By: Fausto, Dimazana, Matuto, Maniquis, Montanno, MalicdemDocument313 pagesEsas Module (Concepts) : Encoded By: Fausto, Dimazana, Matuto, Maniquis, Montanno, MalicdemAlven PullaNo ratings yet
- Detailed Lesson Plan in Science Grade 6Document7 pagesDetailed Lesson Plan in Science Grade 6Mary Joy RobisNo ratings yet
- Mr. Clutter Villegas Middle SchoolDocument9 pagesMr. Clutter Villegas Middle Schoolgilbert_espinosaNo ratings yet
- NSTP CTRL FDocument29 pagesNSTP CTRL FCherie QuintoNo ratings yet
- Cooling Tower Alignment - LaserDocument3 pagesCooling Tower Alignment - LasersyamsidiNo ratings yet
- Pentens Industrial Flooring Solution NewDocument32 pagesPentens Industrial Flooring Solution Newウィリアムズ アンディNo ratings yet
- Fire Is The Rapid Oxidation of A Material in The ExothermicDocument4 pagesFire Is The Rapid Oxidation of A Material in The ExothermicIllavarasanNo ratings yet
- Volume+2 +Table+of+Contents+&+Chapter+17+The+ShiftsDocument36 pagesVolume+2 +Table+of+Contents+&+Chapter+17+The+ShiftsRehan BaigNo ratings yet
- Graham Holton - UFOs and Religious CultsDocument23 pagesGraham Holton - UFOs and Religious CultsJorsant EdNo ratings yet
- Rsos 171773Document17 pagesRsos 171773JULIO CÉSAR CHÁVEZ GALARZANo ratings yet
- 2 Lesson Notes Instrument DatasheetDocument5 pages2 Lesson Notes Instrument Datasheetmubanga20000804100% (1)
- How To Perform Surgical Hand ScrubsDocument4 pagesHow To Perform Surgical Hand ScrubsInna XiiNo ratings yet
- Attachment 1-YICMG2024 Competition ThemeDocument5 pagesAttachment 1-YICMG2024 Competition Themevulananh838No ratings yet