Professional Documents
Culture Documents
Iit Jam Chemical Bonding
Iit Jam Chemical Bonding
Uploaded by
Reedhi KumariOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Iit Jam Chemical Bonding
Iit Jam Chemical Bonding
Uploaded by
Reedhi KumariCopyright:
Available Formats
CHEMICAL BONDING 124
TYPES OF SYMMETRIES OF MO’s
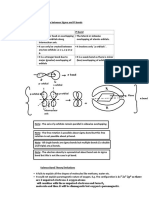
s-s combinations of orbitals:
Atomic Orbitals Molecular Orbitals
+ + +
Bonding orbital
s s (g)
(g)
Node
Antibonding orbital
+ – + – (u)
s s
(u)
s-s combinations of atomic orbitals
In the bonding MO there is increased electron density between the nuclei where as in the anti-bonding MO the
electron density between the nuclui is zero (since the two lobes of opposite sign cancel the function). Both in the
atomic orbital pair before interaction and tthe MO’s formed after interaction, there is cylindrical symmetry
around the bond axis i.e, any point on the orbital surface describes a circle as the MO is rotated around the
bond axis. This circle is a plane perpendicular to the bond axis. All the points on such a circle have either a
positive sign or negative orbital sign. There is no change of sign i.e, no node as we move over the circle. This is
term cyclindrical symmetry and orbitals with cyclindrical symmetry are designated, , if they are bonding and
* , if they are antibonding.
s-p combinatinos of orbitals:
Atomic Molecular
orbitals orbitals
– + + – + overlap
px
bonding orbital
s (g)
Node
+ – + –+ – +
overlap
px
antibonding orbital
s
(u)
s-p combinatino of atomic orbitals
p-p combinations of orbitals:
Molecular
Atomic orbitals
orbitals
– + + – – + – overlap
bonding orbital
px px
(g)
+ – + – + – + – overlap
antibonding orbital
px px
(u)
p-p combinations of atomic orbitals
CHEMICAL BONDING 125
Molecular Orbitals
Atomic Orbitals
+
+ + overlap
Nodal plane bonding orbital
– –
px px
–
(u)
+ – + –
overlap
– Nodal plane antibonding orbital
+
– +
px px
(g)
p-p combinations giving -bonding
p-d combinations of orbitals:
Molecular Orbitals
Atomic Orbitals
+ –
+ + –
overlap
– – bonding orbital
+ +
–
py dyz
(u)
+ – + + –
overlap
antibonding orbital
– + – – +
py dyz
(g)
p-d combinations of atomic orbitals
d-d combinations of orbitals:
–
+
+ + –
–
– +
z
d x2–y2
bonding by d-orbitals
(sideways overlap of two d x2–y2 orbitals)
Non-bonding combinations of orbitals: In the combinations shown in the figure below any stabilization
which occurs from overlapping + with + is destabilized by an equal amount of overlap of + with –. There is no
overall change in energy, and this situation is termed non-bonding. It should be noted that in all these non-
bonding cases the symmetry of the two atomic orbitals is different, i.e. rotation about the axis changes the sign
of one.
You might also like
- 08 Resolver ADocument32 pages08 Resolver AMaria Lunes0% (1)
- Input/output Pole-Placement State-Space Design - Output FeedbackDocument9 pagesInput/output Pole-Placement State-Space Design - Output FeedbackCarlos EduardoNo ratings yet
- GEO Updated-CheatsheetDocument3 pagesGEO Updated-CheatsheetGiovanni LinNo ratings yet
- Coulson MethodDocument17 pagesCoulson MethodSanjeeb SutradharNo ratings yet
- L2 - Chy 222 - 10-01-2024Document25 pagesL2 - Chy 222 - 10-01-2024tedanag282No ratings yet
- Chemical Bonding 03 Class Notes Prayas JEE 2.0 2025Document22 pagesChemical Bonding 03 Class Notes Prayas JEE 2.0 2025ledida5144No ratings yet
- Valency Bond Theory - 013731Document19 pagesValency Bond Theory - 013731ALOK MISHRANo ratings yet
- Chemical Bonding DLPODocument31 pagesChemical Bonding DLPOAyush BoseNo ratings yet
- MergedDocument48 pagesMergedReynald ManaloNo ratings yet
- The If: FingersDocument12 pagesThe If: FingersRiddhi SheteNo ratings yet
- Bonding Slides Aut 2023Document55 pagesBonding Slides Aut 2023aryansrivastava669No ratings yet
- Hydrocarbons Live Class-4 Teacher NotesDocument28 pagesHydrocarbons Live Class-4 Teacher Notesayushgenii1055No ratings yet
- Gen Physics 2 - wORKSHEET 2 - Electric Charge and Electric FieldsDocument4 pagesGen Physics 2 - wORKSHEET 2 - Electric Charge and Electric FieldsDarcy EvansNo ratings yet
- Knowledge: of FpsDocument32 pagesKnowledge: of FpsShreyas PrabhuNo ratings yet
- Lec 1Document12 pagesLec 1sndNo ratings yet
- Hydrogen: EemodeeDocument7 pagesHydrogen: EemodeeGourav SinghNo ratings yet
- 12 AtomsDocument7 pages12 Atomsshivammalik467xNo ratings yet
- Key Unit 2 ReviewDocument4 pagesKey Unit 2 Reviewapi-336093393No ratings yet
- EC Notes Anurag UniversityDocument121 pagesEC Notes Anurag Universityshivamani1489No ratings yet
- Organic Halides Live Class-3 Teacher NotesDocument38 pagesOrganic Halides Live Class-3 Teacher Notessiddhartha singhNo ratings yet
- Coordinate Frames TransformationsDocument15 pagesCoordinate Frames TransformationsH JerryNo ratings yet
- Chemical Bonding (L-5) JLD 3.0Document51 pagesChemical Bonding (L-5) JLD 3.0Ayush BhattacharjeeNo ratings yet
- Flight D. Cheat SheetDocument2 pagesFlight D. Cheat SheetIsaac PittmanNo ratings yet
- QP YEAR - 9 Physics Assessment-1 Statics Electricity RevisedDocument13 pagesQP YEAR - 9 Physics Assessment-1 Statics Electricity RevisedJihan KhanNo ratings yet
- Dielectrics..pptx - PDF Unit III 1Document39 pagesDielectrics..pptx - PDF Unit III 1yazh iniNo ratings yet
- SemiconDocument45 pagesSemiconnardnardNo ratings yet
- Wfra - Me: PBR To RepulsionDocument24 pagesWfra - Me: PBR To RepulsionShreyas PrabhuNo ratings yet
- Nischay MDocument13 pagesNischay MvaibhavNo ratings yet
- CFT NoteDocument22 pagesCFT NoteMuskan BiswalNo ratings yet
- Chemical Bonding.4Document4 pagesChemical Bonding.4VIVEK RASTOGINo ratings yet
- TNID Total Non Dose or DD Displacement Damage: IonizingDocument40 pagesTNID Total Non Dose or DD Displacement Damage: Ionizingtomek_zawistowskiNo ratings yet
- E&M Chap 2 DielectricsDocument33 pagesE&M Chap 2 Dielectricsindomitable7890No ratings yet
- In If BC in - /S' - I5' - : As (C) As (D) - (RF) 21 Be BeDocument1 pageIn If BC in - /S' - I5' - : As (C) As (D) - (RF) 21 Be BejosNo ratings yet
- 665466e124692500181ac8de - ## - Atomic Structure - For - 240602 - 184639Document1 page665466e124692500181ac8de - ## - Atomic Structure - For - 240602 - 184639ayushdhardiwan27No ratings yet
- P72 Lec1Document43 pagesP72 Lec1Allen LoisNo ratings yet
- Properties of Semiconductor: 1. Resistivity 2. Negative Temperature Co-Efficient 3. Conducting Properties (Doping)Document66 pagesProperties of Semiconductor: 1. Resistivity 2. Negative Temperature Co-Efficient 3. Conducting Properties (Doping)Md. Rezwanul IslamNo ratings yet
- Video4 TOM Molecula O2 N2Document8 pagesVideo4 TOM Molecula O2 N2Cx2lhx oneNo ratings yet
- @bohring Bot09 Assignment # EMI & AC Eng@HeyitsyashXDDocument71 pages@bohring Bot09 Assignment # EMI & AC Eng@HeyitsyashXDi.anupamalokNo ratings yet
- Plastic Deformation in Single and Polycrystalline Materials: MSE205:Mechanical Behavior of Materials Pradipta GhoshDocument18 pagesPlastic Deformation in Single and Polycrystalline Materials: MSE205:Mechanical Behavior of Materials Pradipta Ghoshabhishek.kumarNo ratings yet
- R - A Note On The Converse Bricard Property of Projective Planes - SzilasiDocument9 pagesR - A Note On The Converse Bricard Property of Projective Planes - SzilasiUnDueTreSberlaNo ratings yet
- Chapter2 2.PsDocument29 pagesChapter2 2.Psmanjunatha RNo ratings yet
- Goc Revision ALpOMyojEgukEz4EDocument36 pagesGoc Revision ALpOMyojEgukEz4Eanmolsinha6157No ratings yet
- Electric Charge Fundamentals of PhysicsDocument15 pagesElectric Charge Fundamentals of PhysicsMina SamNo ratings yet
- Atomic Structure Part BDocument21 pagesAtomic Structure Part Bxxbackup67No ratings yet
- Isomerisms AA0Document4 pagesIsomerisms AA0IshikaNo ratings yet
- Pure Bending - Multiple Materials & Plastic BendingDocument6 pagesPure Bending - Multiple Materials & Plastic BendingDemi MaNo ratings yet
- Chemic Bonding 3 Yg2LEwNxGBuOW5qvDocument21 pagesChemic Bonding 3 Yg2LEwNxGBuOW5qvrounitraj0987No ratings yet
- Physics Booklet-Pages-945-955Document11 pagesPhysics Booklet-Pages-945-955Aditya VyasNo ratings yet
- Structure of AtomDocument7 pagesStructure of Atomkrarchna115No ratings yet
- Teerahedral: TrigonalDocument1 pageTeerahedral: Trigonalzyzy6527No ratings yet
- Resonance Part 2 PDFDocument21 pagesResonance Part 2 PDFMukul YadavNo ratings yet
- 3emx0 - 2016-06Document3 pages3emx0 - 2016-06stoyan.vercruysseNo ratings yet
- Chemical Bonding Shobhit NirwanDocument17 pagesChemical Bonding Shobhit Nirwanboomb100% (4)
- Chemical Bonding Shobhit NirwanDocument17 pagesChemical Bonding Shobhit NirwanBhavya Goyal XI Non medNo ratings yet
- Conic Sections Formula SheetDocument5 pagesConic Sections Formula Sheetczar czarNo ratings yet
- Unit 6 TrigonometryDocument18 pagesUnit 6 TrigonometryRonny JazakoNo ratings yet
- Success Mathematics SPM Free ChapterDocument22 pagesSuccess Mathematics SPM Free ChapterDaryl Lls100% (1)
- L5 - Chy 222 - 24-01-2024Document14 pagesL5 - Chy 222 - 24-01-2024tedanag282No ratings yet
- Pion-Kaon Femtoscopy in PB PB Collisions at SNN 2.76 TeV Measured With ALICEDocument4 pagesPion-Kaon Femtoscopy in PB PB Collisions at SNN 2.76 TeV Measured With ALICEMuhammad Ibrahim AbdulhamidNo ratings yet
- Candidates Shortlisted For Direct Interview - M.SC - ChemistryDocument1 pageCandidates Shortlisted For Direct Interview - M.SC - ChemistryReedhi KumariNo ratings yet
- PC Rakshit 2-6-12Document7 pagesPC Rakshit 2-6-12Reedhi KumariNo ratings yet
- 108 - Global State of Inclusion - Policy Brief - V21Document24 pages108 - Global State of Inclusion - Policy Brief - V21Reedhi KumariNo ratings yet
- UntitledDocument1 pageUntitledReedhi KumariNo ratings yet
- Notice Board For Updates On Writtent Test and Interviews-Admissions 2024 - 03aprilDocument1 pageNotice Board For Updates On Writtent Test and Interviews-Admissions 2024 - 03aprilReedhi KumariNo ratings yet
- Biochemistry and Cell Biology (Responses) - IInd Internal AssessmentDocument1 pageBiochemistry and Cell Biology (Responses) - IInd Internal AssessmentReedhi KumariNo ratings yet
- End Sem OBE ALS 151 Biocehmistry and Cell Biology - Jun 2021 - ModeratedDocument1 pageEnd Sem OBE ALS 151 Biocehmistry and Cell Biology - Jun 2021 - ModeratedReedhi KumariNo ratings yet
- Textbook Advanced Analytical Methods in Tribology Martin Dienwiebel Ebook All Chapter PDFDocument53 pagesTextbook Advanced Analytical Methods in Tribology Martin Dienwiebel Ebook All Chapter PDFrobert.foster433100% (25)
- Review - 2018 - Final ExamDocument3 pagesReview - 2018 - Final ExamQuang LinhNo ratings yet
- Chemical Energetics Chemistry AS/A LevelDocument4 pagesChemical Energetics Chemistry AS/A Levelyep okNo ratings yet
- Chapter 5 Review AnswersDocument4 pagesChapter 5 Review AnswersMOHA DOOYOWNo ratings yet
- SRM Institute of Science and Technology, Ramapuram Semiconductor Physics (18PYB103J) Unit - IDocument32 pagesSRM Institute of Science and Technology, Ramapuram Semiconductor Physics (18PYB103J) Unit - IRN DpkNo ratings yet
- Structural Ceramic Materials: Boron Carbide - B CDocument7 pagesStructural Ceramic Materials: Boron Carbide - B CMu BaNo ratings yet
- Atomic Physics CIE iGCSE 0625 PPQDocument11 pagesAtomic Physics CIE iGCSE 0625 PPQchelseaNo ratings yet
- Star Q Ipe Chemistry 11th ClassDocument13 pagesStar Q Ipe Chemistry 11th ClassRAYEES UNNISANo ratings yet
- Jorio 2003 New J. Phys. 5 139Document18 pagesJorio 2003 New J. Phys. 5 139Santiago OrtizNo ratings yet
- SCH3U Chemistry Unit 1 MC ReviewDocument16 pagesSCH3U Chemistry Unit 1 MC Review1moeezafNo ratings yet
- Chemical Principles 8th Edition Zumdahl Solutions ManualDocument35 pagesChemical Principles 8th Edition Zumdahl Solutions Manualdement.disturnlklpvp94% (32)
- Schools Division Office I Pangasinan: Republic of The Philippines Department of Education Region IDocument4 pagesSchools Division Office I Pangasinan: Republic of The Philippines Department of Education Region IgarryNo ratings yet
- Introduction To Plant and Power GenerationDocument78 pagesIntroduction To Plant and Power GenerationYoung BrotherNo ratings yet
- Thermochemistry (Chemical Energetics) .: Factors That Affect Enthalpy of Reaction ( )Document36 pagesThermochemistry (Chemical Energetics) .: Factors That Affect Enthalpy of Reaction ( )Nelima Stella mercy100% (1)
- Dose Amd Eq DoseDocument30 pagesDose Amd Eq DoseKhalid AbeedNo ratings yet
- Beta Emitters and Radiation ProtectionDocument7 pagesBeta Emitters and Radiation Protection456 313Lasminar Midauli MNo ratings yet
- End Sem PaperDocument3 pagesEnd Sem PaperHarsh ThakurNo ratings yet
- Inorganic Chem ReviewerDocument4 pagesInorganic Chem ReviewerkezNo ratings yet
- 2022 SPWLA Paper NewPulsedNeutronDocument17 pages2022 SPWLA Paper NewPulsedNeutronDil Dhadakne DoNo ratings yet
- CHM 092 CHAPTER 1 - Matter &stoichiometryDocument128 pagesCHM 092 CHAPTER 1 - Matter &stoichiometryAisyah NadhirahNo ratings yet
- PPSC Chemistry Paper Lecturer 2015 MCQSDocument10 pagesPPSC Chemistry Paper Lecturer 2015 MCQSSana MazharNo ratings yet
- Covalent Bonds, VSEPRDocument6 pagesCovalent Bonds, VSEPRTomáš Tommy NagyNo ratings yet
- ST Xaviers College Entrance Exam PAST Question Papers 7 in Help For SEE AppDocument10 pagesST Xaviers College Entrance Exam PAST Question Papers 7 in Help For SEE AppChij Dhakal100% (1)
- E-Umang - XI JEE - Course PlannerDocument6 pagesE-Umang - XI JEE - Course PlannerRkvNo ratings yet
- Stereochemical Aspects of Organic SynthesisDocument9 pagesStereochemical Aspects of Organic SynthesisNoor NNo ratings yet
- Ws 1b - History of The Atom WorksheetDocument3 pagesWs 1b - History of The Atom WorksheetSofia ShahinNo ratings yet
- Physics 3 For Electrical EngineeringDocument23 pagesPhysics 3 For Electrical Engineering064651922No ratings yet
- Fotokatalitik Pada Permukaan TiO2Document17 pagesFotokatalitik Pada Permukaan TiO2wildaNo ratings yet
- Assignment 7 CHEM216 1Document2 pagesAssignment 7 CHEM216 1Pranav JainNo ratings yet
- s4 Chemistry Paper 1 Mock (2) - 1Document11 pagess4 Chemistry Paper 1 Mock (2) - 1Ndagire OliverNo ratings yet