Professional Documents
Culture Documents
WP Contentuploads202210AQA Topic 1 Key Terms PDF
WP Contentuploads202210AQA Topic 1 Key Terms PDF
Uploaded by
LilyayayOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
WP Contentuploads202210AQA Topic 1 Key Terms PDF
WP Contentuploads202210AQA Topic 1 Key Terms PDF
Uploaded by
LilyayayCopyright:
Available Formats
A Level Biology
TOPIC 1 KEY TERMS AQA Topic 1
Activation energy – amount of energy needed for a Competitive inhibitor – molecule which competes
reaction to begin with the substrate to bind to the active site of
an enzyme
Active site – area of an enzyme where the
specific substrate binds Complementary base pairing – hydrogen bonding
between bases C and G or bases A and T/U
Adenine – base found in DNA, RNA and ATP
Condensation reaction – joins two molecules by
Adenosine diphosphate (ADP) – formed when one
forming a new chemical bond and eliminating a
phosphate group is removed from ATP in a
water molecule
hydrolysis reaction
Cysteine – amino acid containing sulphur
Adenosine triphosphate (ATP) – a nucleotide
derivative, an immediate energy source Cytosine – base found in DNA and RNA
Adhesion – how water molecules are attracted and Denatured – when an enzyme is no longer functional
‘stick’ to other substances e.g xylem vessel walls
Deoxyribonucleic acid (DNA) – double-stranded
Amino acids – monomers of proteins polynucleotide, a type of nucleic acid
Amylopectin – branched α-glucose polymer found in Deoxyribose – pentose sugar found in DNA
plants, a type of starch
Dipeptide – two amino acids joined with a peptide
Amylose – coiled α-glucose polymer found in plants, bond
a type of starch
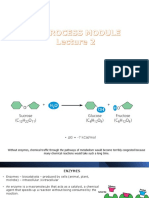
Disaccharide – two monosaccharides joined with a
Antiparallel – when two polynucleotide strands run glycosidic bond e.g sucrose (glucose+fructose),
in opposite directions in DNA lactose (glucose+galactose) and maltose
(glucose+glucose)
ATP hydrolase – enzyme which hydrolyses ATP to
ADP and Pi Disulphide bridge – bond between two sulphur
atoms, important in protein structure
ATP synthase – enzyme which synthesises ATP
from ADP and Pi (condensation reaction) DNA helicase – enzyme which breaks hydrogen
bonds between DNA strands
Base – a nitrogen-containing organic molecule
which is part of a nucleotide DNA polymerase – enzyme which forms
phosphodiester bonds between DNA nucleotides
Benedict’s test – test for sugars
Double helix – twisted shape of DNA, two strands
Biuret test – test for proteins
held together with hydrogen bonds
Cellulose – β-glucose polymer found in plant cell walls
Emulsion test – test for lipids
Cohesion – how water molecules are attracted and
Enzyme – a biological catalyst and soluble protein
‘stick’ to each other using hydrogen bonds
Enzyme-substrate complex – the enzyme plus the
Collagen – a structural protein found in connective
substrate bound to the active site
tissue
Page 1 of 3 © Dr Zoë Huggett
A Level Biology
TOPIC 1 KEY TERMS AQA Topic 1
Ester bond – chemical bond between a fatty acid Latent heat of vaporisation – amount of heat
and glycerol, formed in a condensation reaction energy required for a substance to evaporate
Extracellular – outside a cell Metabolite – a molecule used in a metabolic reaction
Glycerol – molecule found in triglycerides and Monomers – smaller units from which larger
phospholipids molecules (polymers) are made
Glycogen – branched α-glucose polymer found in Monosaccharide – monomers of carbohydrates e.g
animals glucose, fructose and galactose
Glycosidic bond – chemical bond between Non-competitive inhibitor – molecule which binds
monosaccharides, formed in a condensation to an enzyme away from the active site but
reaction changes the shape of the active site
Guanine – base found in DNA and RNA Nucleotides – monomers of nucleic acids (DNA and
RNA)
Hexose sugar – a monosaccharide with six carbon
atoms Pentose sugar – a monosaccharide with five
carbon atoms
Hydrogen bond – attraction between partial
positive and partial negative charges (must Peptide bond – chemical bond between amino acids,
involve hydrogen) formed in a condensation reaction
Hydrolysis reaction – breaks a chemical bond Phosphodiester bond – chemical bond between
between two molecules by inserting a water nucleotides, formed in a condensation reaction
molecule
Phospholipid – lipid similar to a triglyceride but
Hydrophilic – attracts water with one fatty acid substituted with a
phosphate group
Hydrophobic – repels water
Polymers – large molecules made up of large
Inorganic ion – an ion which does not contain
numbers of monomers joined together
carbon (with exceptions)
Polynucleotide – a polymer of many nucleotides
Inorganic phosphate (Pi) – a single phosphate
joined with phosphodiester bonds
group which can be used to phosphorylate other
molecules Polypeptide – a polymer of many amino acids joined
with peptide bonds
Intracellular – within a cell
Polysaccharide – polymer of many
Iodine test – test for starch
monosaccharides joined with glycosidic bonds
Ionic bond – bond between positively and
Protein – one or more polypeptide chains, can have
negatively charged ions, important in protein
different levels of structure
structure
Ribonucleic acid (RNA) – single-stranded
Isomers – molecules with the same molecular
polynucleotide, a type of nucleic acid
formula but a different arrangement of atoms
Page 2 of 3 © Dr Zoë Huggett
A Level Biology
TOPIC 1 KEY TERMS AQA Topic 1
Ribose – pentose sugar found in RNA and ATP Sugar-phosphate backbone – chain of pentose
sugars and phosphate groups joined with
Saturated fatty acid – fatty acid with no double
phosphodiester bonds in a nucleic acid
carbon bonds
Surface tension – formed where water meets air
Semi-conservative replication – DNA replication
due to cohesion
which results in two DNA molecules each
containing one original strand and one new strand Thymine – base found in DNA only
Solvent – a substance which is able to dissolve Triglyceride – lipid formed by joining three fatty
other substances acids to one molecule of glycerol using
condensation reactions
Specific heat capacity – the amount of energy
required to raise the temperature of 1g of a Unsaturated fatty acid – fatty acid with at least
substance by 1°C one double carbon bond
Substrate – molecule complementary to an Uracil – base found in RNA only
enzyme’s active site
Page 3 of 3 © Dr Zoë Huggett
You might also like
- Type Your Name:: Student Guide: Enzymes - AP STEM Case and HandbookDocument5 pagesType Your Name:: Student Guide: Enzymes - AP STEM Case and Handbookanna roth75% (4)
- Macromolecules - Worksheet Answers - OdtDocument4 pagesMacromolecules - Worksheet Answers - OdtRachel JimenezNo ratings yet
- Enzyme Investigation (IA)Document10 pagesEnzyme Investigation (IA)Massimo WuNo ratings yet
- 1020 NotesDocument17 pages1020 NotesAfnan MNo ratings yet
- Physical Science-Finals ReviewerDocument18 pagesPhysical Science-Finals ReviewerPrecious Miracle Lucas SacataniNo ratings yet
- Biomolecules Quizlet Cards PDFDocument18 pagesBiomolecules Quizlet Cards PDFNoura RoseNo ratings yet
- 1610assignment 1 ListDocument7 pages1610assignment 1 Listapi-341205347No ratings yet
- Nucleotide - Monomer of Nucleic AcidsDocument2 pagesNucleotide - Monomer of Nucleic AcidsMika Sophia GonzagaNo ratings yet
- Traffic Light Topic 1 Biological MoleculesDocument3 pagesTraffic Light Topic 1 Biological MoleculesAlison HillNo ratings yet
- Webcontent 146 513 1 Biomolecules 20190926165314Document2 pagesWebcontent 146 513 1 Biomolecules 20190926165314Abhishek Kumar VermaNo ratings yet
- Biological ElementsDocument16 pagesBiological Elementsmarwaaishah01No ratings yet
- Bio Final Review Review Quiz and TestDocument15 pagesBio Final Review Review Quiz and TestLinda ChaoNo ratings yet
- Trans Lecture Intro To BiochemDocument3 pagesTrans Lecture Intro To Biochemlovely coleenNo ratings yet
- Globule: Globular ProteinsDocument1 pageGlobule: Globular ProteinspegasusNo ratings yet
- Chapter 5 VocabDocument2 pagesChapter 5 VocababNo ratings yet
- General Biology 2Document4 pagesGeneral Biology 2Wee-Weh HamjaNo ratings yet
- Biology NotesDocument5 pagesBiology NotesFaith SNNo ratings yet
- Biochem Lec Prelim FinalsDocument11 pagesBiochem Lec Prelim FinalsJullia SollezaNo ratings yet
- Unit 2Document3 pagesUnit 2Kyla Marie PacubasNo ratings yet
- STUDY GUIDE For GENERAL BIOLOGY Finals Google DocsDocument3 pagesSTUDY GUIDE For GENERAL BIOLOGY Finals Google DocsTisha ArtagameNo ratings yet
- General Chemistry 1 - REVIEWERDocument5 pagesGeneral Chemistry 1 - REVIEWEREunice GarciaNo ratings yet
- Definitions Topic 1Document3 pagesDefinitions Topic 1CHRONIKNo ratings yet
- Biochem Final ReviewerDocument19 pagesBiochem Final RevieweryjllhallareNo ratings yet
- BMSCDocument40 pagesBMSCliz.murphy2004No ratings yet
- Biochemistry Week 3 - EnzymesDocument6 pagesBiochemistry Week 3 - EnzymesMicah JadeNo ratings yet
- Gen Biology 2ND MonthlyDocument3 pagesGen Biology 2ND Monthlypearl japsonNo ratings yet
- Physical ScienceDocument6 pagesPhysical ScienceCAYABAN, RISHA MARIE DV.No ratings yet
- Reviwer Sa Gen BioDocument8 pagesReviwer Sa Gen Biocediebanaag10No ratings yet
- Lecture 2Document18 pagesLecture 2warden tambiNo ratings yet
- The Side of Chain of Glycine.: in Ala - Cys - Met - Ser - Tyr, The N - Terminal Is What?Document13 pagesThe Side of Chain of Glycine.: in Ala - Cys - Met - Ser - Tyr, The N - Terminal Is What?John Yman MaglenteNo ratings yet
- Nucleotides & EnzymesDocument16 pagesNucleotides & EnzymesSiti Nur ShafiqahNo ratings yet
- Biochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureDocument4 pagesBiochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureJohn Daniel AriasNo ratings yet
- Chapter 2 - Levels of Organization of The Human BodyDocument9 pagesChapter 2 - Levels of Organization of The Human BodyFrancine Danielle TiponesNo ratings yet
- Biology ASDocument35 pagesBiology ASsalma mazruiNo ratings yet
- HANDOUTSDocument3 pagesHANDOUTScareeseta bongcawilNo ratings yet
- Biochemistry NotesDocument2 pagesBiochemistry Notesnezuko978No ratings yet
- AP Bio Unit 1 Study GuideDocument2 pagesAP Bio Unit 1 Study GuideEllie GriffinNo ratings yet
- BiochemDocument2 pagesBiochemTrixie Myr AndoyNo ratings yet
- Biological MacromoleculesDocument10 pagesBiological MacromoleculesKristine Delos SantosNo ratings yet
- Exam 1 ReviewDocument18 pagesExam 1 ReviewHannah ThorntonNo ratings yet
- Chemical BondsDocument12 pagesChemical BondsjillianneramoranNo ratings yet
- Science Quiz Bee Reviewer - High School LevelDocument6 pagesScience Quiz Bee Reviewer - High School LevelFamella GalmakNo ratings yet
- Energy TransformationDocument5 pagesEnergy TransformationmunozayshiaNo ratings yet
- Genbio 2NDDocument2 pagesGenbio 2NDmamariljasmine03No ratings yet
- Biology ChecklistDocument29 pagesBiology ChecklistgsapkaiteNo ratings yet
- Gen Bio Reviewer Final 1Document5 pagesGen Bio Reviewer Final 1Emmanuelle CalleNo ratings yet
- BIOCHEMISTRY - The Study of The Chemistry of Living: LinkagesDocument2 pagesBIOCHEMISTRY - The Study of The Chemistry of Living: LinkagesOdessa KwonNo ratings yet
- Bio 101 - Integrated Principles of Zoology 18th Edition (Summary of Chapter 2)Document10 pagesBio 101 - Integrated Principles of Zoology 18th Edition (Summary of Chapter 2)Kaeya's asscheeksNo ratings yet
- Microbial Metabolism Metabolism - The Sum of All Chemical ReactionsDocument7 pagesMicrobial Metabolism Metabolism - The Sum of All Chemical ReactionsAGENT M.DNo ratings yet
- Bio ReviewerDocument10 pagesBio ReviewerPau VillanuevaNo ratings yet
- General BiologyDocument17 pagesGeneral Biologydwightballes9No ratings yet
- Cellular/molecular Biology ReviewDocument23 pagesCellular/molecular Biology ReviewStephen ByrneNo ratings yet
- Midterm Biochem ReviewerDocument19 pagesMidterm Biochem ReviewerNATALIE NICOLE GABASNo ratings yet
- SL Option B BiochemistryDocument13 pagesSL Option B Biochemistrypymkmg7c4dNo ratings yet
- Topic 2 Revision NotesDocument5 pagesTopic 2 Revision NotesAldrin TomNo ratings yet
- IBDP - Biology - Chapter 2&7Document10 pagesIBDP - Biology - Chapter 2&7Rafi FarhanNo ratings yet
- Chemical Foundations of BiochemistryDocument5 pagesChemical Foundations of BiochemistryrxpturousNo ratings yet
- ATP ReviewerDocument5 pagesATP ReviewerDaneth Julia TuberaNo ratings yet
- Biochemical Energy Production - BIOCHEMISTRYDocument3 pagesBiochemical Energy Production - BIOCHEMISTRYnezuko978No ratings yet
- Chemical Foundations: Health Maintenance (Nutrition) - Vitamin, Minerals, Water IntakeDocument24 pagesChemical Foundations: Health Maintenance (Nutrition) - Vitamin, Minerals, Water IntakeShyenNo ratings yet
- Enzymes and Vitamins NotesDocument14 pagesEnzymes and Vitamins NotesRealyn ZambasNo ratings yet
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Boron-Based Protease Inhibitors Review Chem Rev 2012Document65 pagesBoron-Based Protease Inhibitors Review Chem Rev 2012Victor CiocalteaNo ratings yet
- Nina StojanovaDocument7 pagesNina StojanovaDespot SinisaNo ratings yet
- Factors Affecting Enzyme ActivityDocument32 pagesFactors Affecting Enzyme ActivitySal KulambaiNo ratings yet
- Tutorial 3 - Biology 101 Answer MemoDocument18 pagesTutorial 3 - Biology 101 Answer MemoKaizer NdoloNo ratings yet
- Edexcel As Biology Revision Notes Unit 1Document15 pagesEdexcel As Biology Revision Notes Unit 1lloyd evansNo ratings yet
- 186 334 1 SM - Id.enDocument15 pages186 334 1 SM - Id.enRizqillahNo ratings yet
- Enzymatic ActivityDocument15 pagesEnzymatic ActivityChristian J. GonzálezNo ratings yet
- Chapter 5 Metabolism and EnzymesDocument8 pagesChapter 5 Metabolism and Enzymes绿谷出久No ratings yet
- Factors Affecting Enzyme ActionDocument18 pagesFactors Affecting Enzyme Actionanon_458882066No ratings yet
- (CC-201) (Microbiology) - 2018Document2 pages(CC-201) (Microbiology) - 2018Sonakshi BafnaNo ratings yet
- 2.5 EnzymesDocument5 pages2.5 EnzymeslhabNo ratings yet
- Bioorganic & Medicinal Chemistry LettersDocument4 pagesBioorganic & Medicinal Chemistry LetterslucasgirioNo ratings yet
- Enzyme InhibitionDocument17 pagesEnzyme InhibitionazwelljohnsonNo ratings yet
- 6728286Document12 pages6728286johan hicoruNo ratings yet
- Introduction To EnymesDocument34 pagesIntroduction To EnymesMeshal NoorNo ratings yet
- BC Review - IDocument11 pagesBC Review - IFlorangNo ratings yet
- Factors Affecting Enzyme ActivityDocument29 pagesFactors Affecting Enzyme ActivityAngela Kim S. BernardinoNo ratings yet
- 02 WholeDocument344 pages02 WholeedithgclemonsNo ratings yet
- CB1 Revision Mat PDFDocument2 pagesCB1 Revision Mat PDFAhmad PatelNo ratings yet
- Enzyme Notes - SathyanarayanaDocument32 pagesEnzyme Notes - SathyanarayanaSwetha RameshNo ratings yet
- SeLnIkRaj - Ligand ScoutDocument26 pagesSeLnIkRaj - Ligand ScoutselnikrajNo ratings yet
- S5 BIOLOGY TEST I TERM II 2024 and MARKING GUIDEDocument16 pagesS5 BIOLOGY TEST I TERM II 2024 and MARKING GUIDEmunyanezaolivier422No ratings yet
- New Inhibitors Targeting Bacterial RNA Polymerase: Seth A. DarstDocument4 pagesNew Inhibitors Targeting Bacterial RNA Polymerase: Seth A. Darstchar462No ratings yet
- Solution 1:: Class XI - NCERT - BiologyDocument9 pagesSolution 1:: Class XI - NCERT - BiologyAdityaNo ratings yet
- Enzyme-Mediated DNA Looping - Halford, Welsh, Szczelkun - 2004Document26 pagesEnzyme-Mediated DNA Looping - Halford, Welsh, Szczelkun - 2004innu88No ratings yet
- Car Boxy Pep Tida SeDocument13 pagesCar Boxy Pep Tida SeJaisy PatelNo ratings yet
- Cambridge International AS & A Level: Biology 9700/21 October/November 2022Document17 pagesCambridge International AS & A Level: Biology 9700/21 October/November 2022Milinda De SilvaNo ratings yet
- EnzymeDocument15 pagesEnzymeAljon Lara ArticuloNo ratings yet