Professional Documents
Culture Documents
Clean-in-Place (CIP) Systems
Clean-in-Place (CIP) Systems
Uploaded by
BramJanssen76Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Clean-in-Place (CIP) Systems
Clean-in-Place (CIP) Systems
Uploaded by
BramJanssen76Copyright:
Available Formats
Facts At Your Fingertips
Clean-in-Place (CIP) Systems
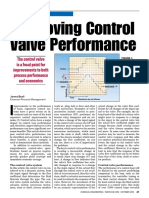
Department Editor: Scott Jenkins CIP supply FIGURE 1. In this typical
C
CIP system, CIP solution
leaning equipment surfaces is ity to drain CIP lines, is sprayed into the tank
critical for processes involving and the appropriate to clean internal surfaces,
biological materials to prevent segregation of the and is drained or pumped
through a separate CIP-
microbial contamination. Clean-in- CIP system from return line
place (CIP) systems play a key role. the process be- Process
vessel
In addition to preventing contamina- ing cleaned. Ideally,
tion, CIP systems also remove grit, dead legs should be
scale and organic matter, which may no longer than two
affect process performance. This pipe diameters, and
one-page reference provides infor- the overall system
mation on CIP system equipment should be designed Process
and operating considerations for to drain completely. CIP return
bioprocessing facilities. Lines should be
sized for fully tur- CIP return pump
CIP equipment bulent flow. The
CIP systems supply fluid to a spray general practice is to have a velocity of the cleaning fluid.
device inside the vessel, which sprays range of 5 to 7 ft/s. All horizontal lines Pre-rinse. The primary objective of
the solution onto the vessel walls. A should be sloped to a drain point, the initial rinse is the mechanical re-
variety of spray devices are available, and low points must be equipped moval of dirt. Water recovered from a
including static sprayballs and fluid- with drains. The minimum slope of later step in the CIP sequence is used
driven orbital cleaners. Sprayballs are the pipe should be at least 1/16 in. for the pre-rinse step. The pre-rinse
high-flow, low-pressure devices often per ft. Valve selection should avoid effluent stream may need to undergo
used to clean tanks smaller than 15-ft non-drainable conditions or crevices a bio-deactivation process before be-
dia., while fluid-driven orbital cleaners that will not be cleaned. So-called ing sent for further waste treatment.
are low-flow, high-pressure devices “clean” ball valve designs are avail- Detergent wash. This step involves
used for tanks greater than 15-ft dia. able for sizes 6 in. and less. For larger chemical cleaning to remove remain-
Tanks. Tanks are typically construct- sizes, hygienic butterfly-valve designs ing dirt. The detergent solution is cir-
ed from 304L or 316L stainless steel. should be considered. The tie-in point culated through the system. The so-
Internal welds should be ground between the CIP system and the pro- lution type and concentration should
smooth and dead spots should be cess should be either a block-and- be determined by plant experience.
minimized. Internal polishing of CIP bleed connection, or a line break. While a 2–4 wt.% caustic solution is
vessels is usually not required. Deter- Instrumentation. Generally recom- commonly used in this step, an acid-
gent tanks should be equipped with mended instrumentation for CIP pro- based detergent (or both) can also be
agitators to ease the preparation of cesses includes the following: used, depending on the type of dirt or
detergent solutions. • Visual sightglasses for CIP supply other contaminants present.
Pumps. There will likely be multiple and return lines Water rinse. A once-through rinse of
unit operations and tanks using the • Temperature indicators on the caus- clean water is typically used, with no cir-
same CIP solutions, but with different tic, acid and rinse-water tanks culation. This substantially reduces the
flow and pressure requirements. To • Conductivity transmitters in the CIP amount of residual materials from the
address this situation, variable-speed supply and return lines detergent wash step. If no acid wash
drives (VSDs) or parallel pumps (sys- • Temperature indication and control is used, this water rinse step becomes
tems with different flows and heads) on the cleaning solution heater the final rinse prior to either sanitization
may be used to meet the range of re- • Temperature indication in the CIP or sterilization. The rinse water should
quirements. Pumps are normally cen- return line be collected for reuse as the pre-rinse
trifugal, often with VSDs. Net positive • Level indicators on all tanks fluid used in the next CIP cycle.
suction head (NPSH) requirements • Differential pressure indicators Acid wash. The solution used in
are an important consideration, due across filters and heat exchangers this step may be circulated in a loop
to the elevated temperatures required • Limit switches confirming position (similar to the detergent wash). This
for some CIP fluids. Hydraulic losses of crucial valves step serves two functions: to neu-
for spray nozzles and equipment tralize and remove any remaining
(heat exchangers, sterilizers and CIP operation caustic from the detergent wash
more) need to be calculated based A typical CIP sequence includes the step; and to remove any hard-water-
on vendor information. following elements: scale deposits that may occur within
Piping. Key considerations of piping Process heel drain. A complete the process equipment. n
design for CIP systems include the drain of the heel is needed to mini-
proper design of CIP circuits, the abil- mize waste and avoid contamination Editor's note: The content for this column was adapted from Miley,
B., Riley, J. and Zelmanovich, Y. Large-Scale Fermentation Systems:
Hygienic Design Principles, Chem. Eng., Nov. 2015. pp. 59–65.
CHEMICAL ENGINEERING WWW.CHEMENGONLINE.COM JUNE 2022 23
You might also like
- Qualification of Purified Water Systems PDFDocument12 pagesQualification of Purified Water Systems PDFDontYou KnowMeNo ratings yet
- YORK Mini VRF ODU - JDOH (040 To 060) - Installation Manual - FAN-1707 201602Document36 pagesYORK Mini VRF ODU - JDOH (040 To 060) - Installation Manual - FAN-1707 201602Douglas Rodriguez100% (1)
- Clean-In-Place (CIP) in Dairy IndustryDocument3 pagesClean-In-Place (CIP) in Dairy IndustryAnil Kumar100% (1)
- CIPguidlineline73707 N3Document9 pagesCIPguidlineline73707 N3Yen NguyenNo ratings yet
- Cleaning in Place (CIP) in Food Processing: December 2013Document55 pagesCleaning in Place (CIP) in Food Processing: December 2013Đivềphía Mặt TrờiNo ratings yet
- ISPE Cleaningvalidation PDFDocument42 pagesISPE Cleaningvalidation PDFAjay KumarNo ratings yet
- How Industrial Businesses Can Reduce Production Costs With Reverse Osmosis: Industrial Reverse OsmosisFrom EverandHow Industrial Businesses Can Reduce Production Costs With Reverse Osmosis: Industrial Reverse OsmosisRating: 5 out of 5 stars5/5 (1)
- Vortex-Breaking PDFDocument7 pagesVortex-Breaking PDFZeroRecoNo ratings yet
- Munich StadiumDocument128 pagesMunich StadiumNikhil BangNo ratings yet
- Oral Presentation Assessment RubricDocument2 pagesOral Presentation Assessment Rubricapi-280384763100% (4)
- A Guide To Clean in Place (CIP)Document6 pagesA Guide To Clean in Place (CIP)Dominic TolentinoNo ratings yet
- A Guide To Clean in Place (CIP) : Your Process System PartnerDocument10 pagesA Guide To Clean in Place (CIP) : Your Process System PartnerKwetishe, Philip ReubenNo ratings yet
- CIPDocument4 pagesCIPpatel909No ratings yet
- Good Cip SystemDocument7 pagesGood Cip SystemThanneeru Naga RajuNo ratings yet
- Reduce Membrane Fouling With Good Cip Procedures inDocument3 pagesReduce Membrane Fouling With Good Cip Procedures indalton2003No ratings yet
- ANATEL Clean-In-Place (CIP) Application NoteDocument8 pagesANATEL Clean-In-Place (CIP) Application NoteMaritza Catalina Melo MartinezNo ratings yet
- Cip CleaningDocument21 pagesCip CleaningNithya CNo ratings yet
- Equipment Cleaning L-45Document3 pagesEquipment Cleaning L-45Bhupendra TomarNo ratings yet
- FOT207 Assignment1Document7 pagesFOT207 Assignment1BRO CODENo ratings yet
- CIP and COP CleaningDocument2 pagesCIP and COP Cleaningamolmob87No ratings yet
- Pump CleaningDocument2 pagesPump CleaningNermeen AhmedNo ratings yet
- CIP CalculationDocument6 pagesCIP CalculationAnonymous fzP6QHQ100% (2)
- GCP Sect17 CIP SystemsDocument17 pagesGCP Sect17 CIP Systemslevanvui161No ratings yet
- Sizing ReceiversDocument3 pagesSizing Receiversjlcheefei9258No ratings yet
- Specifying CIP SystemsDocument15 pagesSpecifying CIP Systemssahirprojects100% (1)
- Strong Base ResinDocument4 pagesStrong Base Resinarvin4dNo ratings yet
- Clean in Place 5 Steps in A Common Cip Cycle InfographicDocument1 pageClean in Place 5 Steps in A Common Cip Cycle InfographicDharanibalan PNo ratings yet
- Pro Cip ManualDocument7 pagesPro Cip Manualmanue2912No ratings yet
- Risk Mitigation For CIP and SIP SystemsDocument4 pagesRisk Mitigation For CIP and SIP Systemsnsk79inNo ratings yet
- Prof. Y.M.Patil: Wastewater Engineering Unit No-3Document37 pagesProf. Y.M.Patil: Wastewater Engineering Unit No-3Akshay JadhavNo ratings yet
- Membrane CleaningDocument5 pagesMembrane CleaninggmarinovNo ratings yet
- Europe EEMEA CIP Pest Management Dairy Suppliers 2015 PDFDocument92 pagesEurope EEMEA CIP Pest Management Dairy Suppliers 2015 PDFkunal shahNo ratings yet
- GCB 2009 Sect16 CIP SystemsDocument17 pagesGCB 2009 Sect16 CIP SystemsRiyanNo ratings yet
- Revised Pacifica Bandini Engineering's ReportDocument10 pagesRevised Pacifica Bandini Engineering's ReportGarry PonferradaNo ratings yet
- Bic Dynamic SBR SystemsDocument6 pagesBic Dynamic SBR SystemsViorel HarceagNo ratings yet
- CIP Optimization: Hyde Engineering OverviewDocument24 pagesCIP Optimization: Hyde Engineering OverviewbioNo ratings yet
- Automation of Cip Process in Dairy Industries Using Programmable Controllers and ScadaDocument6 pagesAutomation of Cip Process in Dairy Industries Using Programmable Controllers and ScadaLita RaniNo ratings yet
- Cleaning in Place (CIP)Document15 pagesCleaning in Place (CIP)Sanjay v.r v.rNo ratings yet
- Caustic OptimizationDocument4 pagesCaustic OptimizationNafees KhanzadaNo ratings yet
- Chemical Clean in Place (CIP)Document8 pagesChemical Clean in Place (CIP)Shashikant PanchalNo ratings yet
- Revised Royal Bellagio Engineering's ReportDocument12 pagesRevised Royal Bellagio Engineering's ReportGarry PonferradaNo ratings yet
- Cleaning in The Life Science Industry - TACCT BPE v2.0Document45 pagesCleaning in The Life Science Industry - TACCT BPE v2.0ken1962No ratings yet
- CIP Challenges in DairyDocument9 pagesCIP Challenges in DairyFaisal MustafaNo ratings yet
- Acid Cleaning ProcedureDocument6 pagesAcid Cleaning ProcedureMadhan RajNo ratings yet
- CIP Cleaning MachineDocument13 pagesCIP Cleaning MachineCoteneanuIonutNo ratings yet
- Flushing, Cleanning and Desinfection of The Domestic Water SystemDocument7 pagesFlushing, Cleanning and Desinfection of The Domestic Water Systembani alsharifNo ratings yet
- 40 - Suncombe CIP Overview PresentationDocument46 pages40 - Suncombe CIP Overview PresentationSantiago AguayoNo ratings yet
- Automated Cleaning in Dairy Industry Using CIP MethodDocument4 pagesAutomated Cleaning in Dairy Industry Using CIP MethodInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Sewage Treatment Plant Report For M/s Santla Devi Resorts Pvt. LTDDocument7 pagesSewage Treatment Plant Report For M/s Santla Devi Resorts Pvt. LTDSamNo ratings yet
- Technical Information CIP COPDocument10 pagesTechnical Information CIP COPyosep naibahoNo ratings yet
- Gea - STD Single Use Cip Sys - CipdDocument6 pagesGea - STD Single Use Cip Sys - CipdTBNo ratings yet
- Gea Cip Star System Brewhouse 296991Document4 pagesGea Cip Star System Brewhouse 296991Hoàng Nguyễn VănNo ratings yet
- CIP InfoSheetPharmaDocument2 pagesCIP InfoSheetPharmaENRIQUE_POMALES683No ratings yet
- Biotechnology Inspection Guide Reference Materials and TraDocument2 pagesBiotechnology Inspection Guide Reference Materials and TraGirgis AiadNo ratings yet
- Criticial Utilities Qualifcation Part IIDocument12 pagesCriticial Utilities Qualifcation Part IInavas1972No ratings yet
- Cip and Designing of SystemDocument4 pagesCip and Designing of SystemMonty KushwahaNo ratings yet
- BatchpurWWTP2022 ADocument8 pagesBatchpurWWTP2022 AKshitiz MittalNo ratings yet
- 01.22.16 Coca Cola Process DescriptionDocument32 pages01.22.16 Coca Cola Process DescriptionHausland Const. Corp.No ratings yet
- ArticleDocument7 pagesArticleRamachandra Bhat HireNo ratings yet
- Cip / Sip: IWI EDocument4 pagesCip / Sip: IWI EPaco Daniel Caastillo SalgadoNo ratings yet
- 4 - Best Practices For Sodium Hypochlorite Storage and Metering SystemsDocument48 pages4 - Best Practices For Sodium Hypochlorite Storage and Metering SystemsZain Ali100% (1)
- CIP Drying PlantDocument8 pagesCIP Drying Planta_roghibNo ratings yet
- Water Treatment Plant Performance Evaluations and OperationsFrom EverandWater Treatment Plant Performance Evaluations and OperationsNo ratings yet
- Harvesting Rainwater for Your Home: Design, Install, and Maintain a Self-Sufficient Water Collection and Storage System in 5 Simple Steps for DIY beginner preppers, homesteaders, and environmentalistsFrom EverandHarvesting Rainwater for Your Home: Design, Install, and Maintain a Self-Sufficient Water Collection and Storage System in 5 Simple Steps for DIY beginner preppers, homesteaders, and environmentalistsNo ratings yet
- An Introduction To Canned Motor PumpsDocument4 pagesAn Introduction To Canned Motor PumpsBramJanssen76No ratings yet
- Bolted Flange Joint AssembliesDocument1 pageBolted Flange Joint AssembliesBramJanssen76No ratings yet
- Applying Location Factors For Conceptual Cost EstimationDocument3 pagesApplying Location Factors For Conceptual Cost EstimationBramJanssen76100% (1)
- A Brief Review of Natural Water's Influence On Scale Formation in Heat ExchangersDocument4 pagesA Brief Review of Natural Water's Influence On Scale Formation in Heat ExchangersBramJanssen76No ratings yet
- Building Better Seal-Support Systems For PumpsDocument3 pagesBuilding Better Seal-Support Systems For PumpsBramJanssen76No ratings yet
- An Overview of Non-Combustion Clean Air TechnologiesDocument3 pagesAn Overview of Non-Combustion Clean Air TechnologiesBramJanssen76No ratings yet
- New Membrane Applications For Traditional Water-Treatment ProcessesDocument4 pagesNew Membrane Applications For Traditional Water-Treatment ProcessesBramJanssen76No ratings yet
- Can The Cure Be As Bad As The IllnessDocument1 pageCan The Cure Be As Bad As The IllnessBramJanssen76No ratings yet
- Can Trash Interfere With A CureDocument2 pagesCan Trash Interfere With A CureBramJanssen76No ratings yet
- Assessment Protocol For Nozzle Loads On Pressure VesselsDocument5 pagesAssessment Protocol For Nozzle Loads On Pressure VesselsBramJanssen76No ratings yet
- Lessons Learned in The Classroom - Tower Pressure and CapacityDocument1 pageLessons Learned in The Classroom - Tower Pressure and CapacityBramJanssen76No ratings yet
- Avoiding MIstakes When Emptying Spill PalletsDocument2 pagesAvoiding MIstakes When Emptying Spill PalletsBramJanssen76No ratings yet
- Valves - Essential WorkhorsesDocument2 pagesValves - Essential WorkhorsesBramJanssen76No ratings yet
- Milling in The Pharmaceutical IndustryDocument7 pagesMilling in The Pharmaceutical IndustryBramJanssen76No ratings yet
- Valve Actuator Selection GuideDocument3 pagesValve Actuator Selection GuideBramJanssen76100% (1)
- Advances in Chlor-Alkali TechnologiesDocument3 pagesAdvances in Chlor-Alkali TechnologiesBramJanssen76No ratings yet
- Vent Away Condensate Pump Frustrations in A FlashDocument6 pagesVent Away Condensate Pump Frustrations in A FlashBramJanssen76100% (1)
- Wastewater Treatment - Three Steps To Achieving Discharge ComplianceDocument2 pagesWastewater Treatment - Three Steps To Achieving Discharge ComplianceBramJanssen76No ratings yet
- Safety Relief Valves - Installation and MaintenanceDocument3 pagesSafety Relief Valves - Installation and MaintenanceBramJanssen76No ratings yet
- Toward The Production of Safer ChemicalsDocument5 pagesToward The Production of Safer ChemicalsBramJanssen76No ratings yet
- Why Innovation Operations Are CriticalDocument4 pagesWhy Innovation Operations Are CriticalBramJanssen76No ratings yet
- Velocity of Ultrasound in Commonly Used Vegetable Oils at Low FrequenciesDocument7 pagesVelocity of Ultrasound in Commonly Used Vegetable Oils at Low FrequenciesBramJanssen76No ratings yet
- Unlocking Hydraulic Limits in A RevampDocument7 pagesUnlocking Hydraulic Limits in A RevampBramJanssen76No ratings yet
- Velocity of Sound in Vegetable OilsDocument3 pagesVelocity of Sound in Vegetable OilsBramJanssen76No ratings yet
- If Data Is The New Gold, Where To Start DiggingDocument6 pagesIf Data Is The New Gold, Where To Start DiggingBramJanssen76No ratings yet
- The Importance of Steam Quality For Steam-System Process OperationDocument4 pagesThe Importance of Steam Quality For Steam-System Process OperationBramJanssen76No ratings yet
- Ultrasonic Studies of Palm Oil and Other Vegetable OilsDocument8 pagesUltrasonic Studies of Palm Oil and Other Vegetable OilsBramJanssen76No ratings yet
- Improving Control Valve PerformanceDocument5 pagesImproving Control Valve PerformanceBramJanssen76No ratings yet
- Health, Safety and Environmental Considerations For Process SynthesisDocument6 pagesHealth, Safety and Environmental Considerations For Process SynthesisBramJanssen76No ratings yet
- The Production of Organic Manure Using Kitchen WasteDocument27 pagesThe Production of Organic Manure Using Kitchen WasteAabhas RathNo ratings yet
- Inversor Automatico de Fuente de Poder Masterpact UA-BADocument38 pagesInversor Automatico de Fuente de Poder Masterpact UA-BARodolfoAntonioLeónCárdenas100% (1)
- Food Industry and Processing Technology: On Time To Harmonize Technology and Social DriversDocument13 pagesFood Industry and Processing Technology: On Time To Harmonize Technology and Social DriversOctaverina Rezki TamaraNo ratings yet
- The First True Turnkey Solution For Landbased Coastal SurveillanceDocument2 pagesThe First True Turnkey Solution For Landbased Coastal SurveillancefrcantarinoNo ratings yet
- Test MCCDocument4 pagesTest MCCBogdan TudoricaNo ratings yet
- Ti-Spc-Psi-Protct-6070 (9 08) PDFDocument49 pagesTi-Spc-Psi-Protct-6070 (9 08) PDFsrdeetrdsbc100% (2)
- Crude Oil TreatmentDocument27 pagesCrude Oil TreatmentmahmoudNo ratings yet
- Rojkind ArquitectosDocument6 pagesRojkind ArquitectosFabrizio AlbertoNo ratings yet
- DSR - Dec. 26, 2022 - Jan. 8, 2023Document6 pagesDSR - Dec. 26, 2022 - Jan. 8, 2023BuenafeNo ratings yet
- RGB Fan Connection TranslatedDocument30 pagesRGB Fan Connection Translatedjonel.javierNo ratings yet
- Muhammed Suhail K: Site Engineer Cum Safety OfficerDocument2 pagesMuhammed Suhail K: Site Engineer Cum Safety Officermsksuhail 8No ratings yet
- Result-Based Performance Management System (RPMS) : PortfolioDocument19 pagesResult-Based Performance Management System (RPMS) : PortfolioNota Belz100% (4)
- ELVIC Superwinch Hidraulico Manual ModelosH8P H10P PRODocument12 pagesELVIC Superwinch Hidraulico Manual ModelosH8P H10P PROorlinNo ratings yet
- An Interactive EDocument19 pagesAn Interactive Etesfu100% (1)
- Cork Chamber - Chamberlink (Nov 2013)Document24 pagesCork Chamber - Chamberlink (Nov 2013)Imelda V. MulcahyNo ratings yet
- Educational Information For High Expansion Foam Fire Extinguishing SystemDocument3 pagesEducational Information For High Expansion Foam Fire Extinguishing SystemAjay W DhimanNo ratings yet
- Planning The New VentureDocument17 pagesPlanning The New VentureStoryKingNo ratings yet
- LM2500 AssessmentDocument3 pagesLM2500 AssessmentKALPUSH50% (2)
- Ac DC LoadflowDocument35 pagesAc DC LoadflowSajith RpNo ratings yet
- Fault StudyDocument12 pagesFault StudypasanhesharaNo ratings yet
- Internet Broadband Zambia, International Call Bundles ZambiaDocument3 pagesInternet Broadband Zambia, International Call Bundles ZambiaMTN ZambiaNo ratings yet
- Relational Algebra: Operators Expression TreesDocument28 pagesRelational Algebra: Operators Expression TreesYatin NimeshNo ratings yet
- FortiGate Example SOHO 01-30006-0062-20080310Document54 pagesFortiGate Example SOHO 01-30006-0062-20080310Bijay ShakyaNo ratings yet
- Agma Gear Rating Suite 3Document1 pageAgma Gear Rating Suite 3mgualdiNo ratings yet
- EMS-BGF Windows IG V13.5 en PDFDocument40 pagesEMS-BGF Windows IG V13.5 en PDFCount_ZNo ratings yet
- EzyMesh and Accessories DataSheetDocument2 pagesEzyMesh and Accessories DataSheet30101985No ratings yet