Professional Documents
Culture Documents
Show The Mechanism For The Reaction of Acetic Anh
Show The Mechanism For The Reaction of Acetic Anh
Uploaded by
Noor FarhanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Show The Mechanism For The Reaction of Acetic Anh
Show The Mechanism For The Reaction of Acetic Anh
Uploaded by
Noor FarhanCopyright:
Available Formats
Log In Menu
Science
Show the mechanism for the
reaction of acetic anhydride
with water.
Question:
Show the mechanism for the reaction of acetic
anhydride with water.
Hydration Reaction
The reaction of any compound with water is known
as hydration reaction. The water molecule acts as
nucleophile and attack at the electrophilic carbon
center. The acetic anhydride undergoes hydration
reaction to form acetic acid.
Answer and Explanation:
The reaction of acetic anhydride with water occurs in
four steps, which are stated below.
Step:1In the !rst step, the lone present on the
oxygen atom of water attacks the electrophilic
carbonyl carbon atom of acetic anhydride. The
double bond electron density moves to the oxygen
atom.
Step:2The removal of a proton from the resulting
compound takes place.
Step:3The carbonyl double bond electron density
moves back to the carbon atom and carbon-oxygen
double bond is formed, simultaneously the carbon-
oxygen single bond is broken. This results in the
formation of acetic acid and acetate ion.
Step:4In the !nal step of the reaction, the acetate ion
takes proton to form acetic acid.
The complete mechanism for the reaction of acetic
anhydride with water is shown below.
Reaction of acetic anhydride with water
Therefore, the reaction of acetic anhydride with
water forms two molecules of acetic acid.
Help improve Study.com. Report an Error
Become a member and unlock all
Study Answers
Start today. Try it now
Create an account
Ask a question
Our experts can answer your tough homework and
study questions.
Ask a question
Search Answers
What question do you need help with?
Learn more about this topic:
What is Hydration? - De!nition, Facts & Bene!ts
from
Chapter 26 / Lesson 8
70K
Why is it important to drink water? See the de!nition of
hydration, read about the hydration process and bene!ts of
hydration, and learn how to stay hydrated.
Related to this Question
Provide a detailed mechanism to show reaction for acetic
anhydride with water?
Please show the chemical reaction for 4 aminophenol with
acetic anhydride to produce acetaminophen.
Write a mechanism for the reaction of p-toluidine with acetic
anhydride. Why is sodium acetic added to this reaction?
Propose a mechanism for the reaction of benzoyl chloride
with acetic acid, and show the structure of the resulting
anhydride.
Write the mechanism of the reaction between p-
hydroxyaniline and acetic anhydride to prepare
acetaminophen.
Draw the product and mechanism for the esteri!cation
reaction of acetic anhydride and 1-octanol.
In this experiment excess acetic anhydride is converted to
acetic acid when water is added to the reaction product. Write
an equation for this reaction.
Write a mechanism for the reaction of p-toluidine with acetic
anhydride. Why is sodium acetate added to this reaction?
Draw the reaction between 4-Nitrophenol and acetic
anhydride as well as the anticipated product(s).
What is the reaction mechanism to form N-acetylanthranilic
acid from anthranilic acid and acetic anhydride? Show where
to push the arrows.
Demonstrate the reaction mechanism by which pentane-1,5-
dial (glutaraldehyde) is transformed into succinic anhydride
under acidic conditions (H3O+).
Vanillin is mixed with acetic anhydride in the present of NaOH
to form an ester. Draw out the mechanism of the reaction.
Draw a synthesis of phenacetin that employs acetic
anhydride, with mechanism.
Draw the product and mechanism for the esteri!cation
reaction of acetic anhydride and 1-octanol (assuming there is
an acid catalyst present).
Draw the product and mechanism for the esteri!cation
reaction of acetic anhydride and 1-octanol (assuming there is
acid catalyst present).
Show all reactants, reaction conditions in brackets and
products for the production of acetic acid from chloroethane
Draw the reaction between propanol and acetic anhydride as
well as the anticipated product(s).
Write the structure of the product that would form from the
following reaction. Phenol + Acetic anhydride.
Show the mechanism of the following reaction: NaOH
2XeF2 + 2H2O = 2Xe + 4HF + O2 Show reaction mechanism of
the following reaction.
How do you draw the arrow pushing mechanism for the
formation of the acylium ion when acetic anhydride reacts
with phosphoric acid?
Show the full mechanism for the esteri!cation of acetic acid
with octyl alcohol.
Please show a mechanism for each reaction.
Show a mechanism for the reaction.
Show the mechanism for the reaction below.
Water has no e#ect on the yield because it destroys both
acetic anhydride and aspirin.
1. a) The name acetic anhydride implies that the compound
will react with water to form acetic acid. Write the equation
for the reaction. b) Identify R and R' in Equation 1 when the
ester, aspirin, i
Show the mechanism of the reaction below and give the
structure of the product.
Show the hydrolysis of the product 9,10-dihydroanthracene-
9,10-succinic anhydride.
When synthesizing aspirin, using acetic anhydride, acetic
anhydride is reacting as if it were a/an _____________.
If a student used acetic acid in place of acetic anhydride when
synthesizing acetaminophen, what reaction would occur?
Draw the product.
Show the mechanism for the two reactions:
What is the product of the following modi!ed Perkin reaction:
3-benzoylpropionic acid + benzaldehyde + sodium acetate +
acetic anhydride when heated to 100 degrees Celsius? Show
the mechanism.
Give the mechanism for the reaction of cyclopentadiene with
maleic anhydride (Diels Adler reaction).
a) The name acetic anhydride implies that the compound will
react with water to form acetic acid. Write the equation for
the reaction. b) Write the equation for the reaction by which
aspirin decomposes in an aqueous ethanol solution.
Show the mechanism and product of this reaction.
How could you perform the following crossed-Aldol reaction
to obtain the product selectively? Show the mechanism. A.
Use sodium methoxide B. Use potassium tert-butoxide. C.
Use acetic acid. D. Use
Show a mechanism for the following reaction.
Show the mechanism of the following reaction:
Write out the reaction and mechanism for the synthesis of
phenacetin from acetic anhydride and p-phenetidine via
amide synthesis.
Show the mechanism for the reaction of RUBISCO with CO_2
and H_2O. CH_2OPO_3CHOHCHOHCHOHCH_2OPO_3 + CO_2
+ H_2O
Show all steps in the mechanism for the reaction:
CH_3C=OCH_2CH_3 + H_2O overset{H_2SO_4}{rightarrow}
Show product and mechanism of the reaction.
Show the product and the mechanism for this reaction.
Write the structure of the product(s) that you would expect
from the following reaction: Phenol + Acetic anhydride. Also,
write a complete reaction.
Please show the mechanism and product of the following
reaction.
Show the mechanism and product for the following reaction.
Explain why trichloroacetic anhydride ((Cl_3CCO)_2O) is more
reactive than acetic anhydride ((CH_3CO)_2O) in nucleophilic
acyl substitution reactions.
Illustrate the mechanism involved in the synthesis of aspirin
without utilizing an acid catalyst. Although a metal catalyst is
used in this green chemistry synthesis. The reactants involved
in this reaction are salicylic acid and acetic anhydride.
Na2Cr2O7/acetic acid is used for reaction.
Show the complete mechanism of this Aldol reaction.
Show a detailed reaction mechanism for the following
reaction.
Please show the mechanism of the following reactions.
Give a detailed mechanism (show electron $ow) for the Diels-
Alder reaction for the synthesis of cis-norbornene-5,6-endo-
dicarboxylic anhydride from cyclopentadiene and maleic
anhydride.
Show the mechanism, and draw the products of the following
reaction.
Show the mechanism for this substitution reaction (SN2)
Find the reagents for this reaction to happen and show the
mechanism.
Draw the major organic product from the reaction of 2-
(carboxymethyl)benzoic acid with acetic anhydride and heat.
Show the esteri!cation reaction which produces ethyl formate
and the esteri!cation process which produces ethyl butyrate.
What are the products of the reaction of acetic anhydride
with: 1. diphenylamine 2. water (with heat) 3. benzene in the
presence of a lewis acid 4. toluene in the presence of a lewis
acid 5. ethanol (
Show the mechanism of this reaction: (Image)
Draw the mechanism for the reaction shown.
Show the mechanism for the given reaction below. What does
the mechanism have in common? Why?
Show the mechanism for the given reaction. What does the
mechanism have in common? Why?
Show the mechanism for the reaction of cyclohexene with
HCl.
Aspirin (C_9H_8O_4) is produced by the reaction of salicylic
acid (C_7H_6O_3, M = 138.1 g/mol) and acetic anhydride
(C_4H_6O_3, M = 102.1 g/mol): C_7H_6O_3 (s) + C_4H_6O_3 (l)
\to C_9H_8O_4 (s) + C_2H_4O_2 (l) If you react 2.00 g
C_7H_6O_3 with 1.60 g C_4
Aspirin (C_9H_8O_4) is produced by the reaction of salicylic
acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol).
C_7H_6O_3 + C_4H_6O_3 ---> C_9H_8O_4 + C_2H_4O_2 If you
react 2.00 g C_7H_6O_3 with...
Show the mechanism for the following reaction below. What
does the mechanism have in common? Why?
Show the product of the following reactions with mechanism.
Write the reaction equation showing structural formulas for
the formation of an ester from 2-methyl-1-butanol and acetic
acid.
Show the chemical reaction for the hydrolysis of the ester,
ethyl acetate, indicating the two products of this reaction.
1) Write a reaction to show how a sodium acetate/acetic acid
bu#er would respond to a small amount of added strong
acid. 2) Write a reaction to show how a sodium acetate/acetic
acid bu#er would resp
Find the product of the following reaction and show its
mechanism.
In the synthesis of aspirin we react salicylic acid
(HOOCC_6H_4OH) with acetic anhydride (C_4H_6O_3). The
unbalanced chemical equation is: HOOCC_6H_4OH +
C_4H_6O_3 to HOOCC_6H_4O_2C_2H_3 + H_2O. If we
Draw the organic product formed by estradiol when it reacts
with excess acetic anhydride in pyridine.
Draw a stepwise mechanism for the shown reaction.
Draw a stepwise mechanism for the shown reaction below.
Write out the reaction and mechanism for the synthesis of
phenacetin from acetic anhydride and phenetidine (p-
ethoxyaniline) via amide synthesis (a nucleophilic acyl
substitution).
Show the mechanism of the Wittig reaction in steps and
explain each step.
Show the mechanism of nitration of benzene using Friedel-
crafts reaction.
Can someone help me draw the complete reaction
mechanism for bromination of trans-cinnamic acid using
pyridinium tribromide and glacial acetic acid??? I can't !gure it
out. I tried the mechanism for
Aspirin can be made from the reaction between a phenol and
an anhydride. What is the reaction mechanism for this?
Show the complete reaction for preparing isoamylacetate,
using arrows to indicate electron $ow. Isoamyl alcohol +
acetic acid isoamyl acetate
Depict the mechanism of the reaction.
Write an equation for the reaction of one molecule of
ethylene glycol with one molecule of phthalic anhydride.
Show the step by step mechanism for the reaction:
The reaction between salicylic acid and acetic anhydride,
which is catalyzed by sulfuric acid and produces aspirin and
acetic acid is given. Write an equation for a reaction that
might form salicylic acid during the workup of the product.
Show the reagent and mechanism for the reaction of
methylene cyclohexane given below.
Show a reaction, which illustrates the hydrolysis of
acetylsalicylic acid.
Show the reaction from cyclopentanoic acid to cyclohexanoic
acid .
Write the equation showing the structures of the reactants
and products in the reaction of acetanilide with bromine in
acetic acid.
The triene shown here reacts with 2 equivalents of maleic
anhydride to yield a product with the formula C17H16O6.
Predict a structure for the product.
Complete the reaction. Show the mechanism.
When wine spoils, ethanol is converted to acetic acid. The
following reaction illustrates this process. C 2 H 5 O H ( l ) + O
2 ( g ) C H 3 C O O H ( l ) + H 2 O ( l ) Given that H = 492.5 k J
and S = 136.1 k J , calculate G at 25
In the reaction p-aminophenol + acetic anhydride arrow
acetaminophen + acetic acid, is it true that acetic acid is
formed as a by-product by the reaction between the unused
acetyl group of acetic anhydride and the hydrogen ion lost
from the amine group of
Show the mechanisms of these two reactions.
Draw the mechanism for the following reaction. Please show
and explain all the steps.
Show a mechanism for the following reaction. Assume the
given product is the only product.
Give a step-by-step mechanism for the reaction of the
following: Salicylic acid with acetic anhydride/ phosphoric acid
Aspirin can be made in the laboratory by reacting acetic
anhydride (C_4H_6O_3) with salicylic acid (C_7H_6O_3) to form
aspirin (C_9H_8O_4) and acetic acid (C_2H_4O_2). The
balanced equation is: C_4H_6O_3+C_7H_6O_3 \rightarrow
C_9H_8O_4+C_2H_4O_2. In a l
Explore our homework questions
and answers library
Search
What question do you need help with?
Browse
Browse by subject
© copyright 2003-2023 Homework.Study.com. All other trademarks
and copyrights are the property of their respective owners. All rights
reserved.
Resources and Guides About Us Terms of Use Privacy Policy
DMCA Notice ADA Compliance Honor Code For Students
You might also like
- Experiment 8 - The Preparation of AcetanlideDocument12 pagesExperiment 8 - The Preparation of AcetanlideMark Ryan Tripole92% (13)
- B62 0300 (Rev. B 2004.08) EN - THERMOPLASTIC, THERMOSETTING MATERIALS THERMOPLASTIC ELASTOMERS AND RUBBERS - CLASSIFICATIONDocument20 pagesB62 0300 (Rev. B 2004.08) EN - THERMOPLASTIC, THERMOSETTING MATERIALS THERMOPLASTIC ELASTOMERS AND RUBBERS - CLASSIFICATIONDiego CamargoNo ratings yet
- Production of MethylacetateDocument57 pagesProduction of MethylacetateAhmed Ali50% (2)
- Classification Tests For Carboxylic Acids and Their DerivativesDocument9 pagesClassification Tests For Carboxylic Acids and Their DerivativesAngelyka Cabalo100% (1)
- Preparation of Butyl Acetate PDFDocument6 pagesPreparation of Butyl Acetate PDFjoiya100133% (3)
- Experiment 13Document6 pagesExperiment 13Anna Sophia EbuenNo ratings yet
- Project 1 - Isopropanol and Acetone From Propylene PDFDocument8 pagesProject 1 - Isopropanol and Acetone From Propylene PDFAnonymous RJkpep7D0rNo ratings yet
- Exp 8 - Abstract, Intro & AtqDocument6 pagesExp 8 - Abstract, Intro & AtqChali HaineNo ratings yet
- Esterification Salicylic AcidDocument3 pagesEsterification Salicylic AcidBobbyGunarsoNo ratings yet
- CH 16 PracticeDocument8 pagesCH 16 Practiced_denbergNo ratings yet
- 123Document6 pages123Julius Rafael Delprado DildigNo ratings yet
- Synthesis of Isopentyl AcetateDocument6 pagesSynthesis of Isopentyl AcetateAmiratul FazirahNo ratings yet
- Jasmin Malhotra - Esters PracDocument7 pagesJasmin Malhotra - Esters Pracapi-287665202100% (2)
- 360exp10-02 EsterificationDocument14 pages360exp10-02 EsterificationlewisrahimiNo ratings yet
- 5-Synthesis of EstersDocument6 pages5-Synthesis of EstersPeter YekNo ratings yet
- Lab 10 N Acetylation - The Acetylation of A Primary Aromatic AmineDocument7 pagesLab 10 N Acetylation - The Acetylation of A Primary Aromatic Aminekiran_1792No ratings yet
- LAB QO 1 - P-MethoxyacetanilideDocument17 pagesLAB QO 1 - P-MethoxyacetanilidemarioNo ratings yet
- AldolDocument7 pagesAldolGindo Baroes WanNo ratings yet
- T H E Synthesis of Acid and Related Compounds1: Indole-3-Acetyl-D, L-AsparticDocument3 pagesT H E Synthesis of Acid and Related Compounds1: Indole-3-Acetyl-D, L-AsparticDuygu TürkyılmazNo ratings yet
- Lecture Questions CZB190Document18 pagesLecture Questions CZB190micro0908No ratings yet
- Exp 10 EsterDocument14 pagesExp 10 EsterLaris J. Garcia100% (1)
- Lab Activity 10 (Organic Chem)Document2 pagesLab Activity 10 (Organic Chem)christina lepitenNo ratings yet
- Nucleophlic Acyl Substitution - The Synthesis of Ethyl ButanoateDocument5 pagesNucleophlic Acyl Substitution - The Synthesis of Ethyl ButanoateRuther Cabral67% (3)
- Organic Chemistry - Esters Lab & Lab Report (Making Scents of Esters)Document7 pagesOrganic Chemistry - Esters Lab & Lab Report (Making Scents of Esters)Mark Riley86% (14)
- FileDocument8 pagesFileKhairil AnshariNo ratings yet
- Methyl Benzoate Prac 2Document16 pagesMethyl Benzoate Prac 2Patience Paida NganiNo ratings yet
- 210 - 1,4-Di-T-Butyl-2,5-Dimethoxy Benzene and Acetanilide - Sp. 2023Document27 pages210 - 1,4-Di-T-Butyl-2,5-Dimethoxy Benzene and Acetanilide - Sp. 2023Abdallah EliasNo ratings yet
- Chem - Expt 10Document4 pagesChem - Expt 10Mirzi TurbolenciaNo ratings yet
- Functional Groups: Naming of EstersDocument6 pagesFunctional Groups: Naming of EsterspappadakunduNo ratings yet
- Acetaldehyde Report - Final PDFDocument20 pagesAcetaldehyde Report - Final PDFDinesh guhanNo ratings yet
- ExerciseDocument16 pagesExercisecse.220840131017No ratings yet
- Aldol Notes PDFDocument8 pagesAldol Notes PDFAna100% (1)
- BenzoicDocument6 pagesBenzoicIsmail çıtakNo ratings yet
- Topical and Prospective Processes of Acetoxylation: Grzegorz Lewandowski, Marcin Bartkowiak, Eugeniusz MilchertDocument6 pagesTopical and Prospective Processes of Acetoxylation: Grzegorz Lewandowski, Marcin Bartkowiak, Eugeniusz MilchertAnonymous b9fcR5No ratings yet
- Question Bank On Aldehydes Ketiones Carboxylic AcidsDocument3 pagesQuestion Bank On Aldehydes Ketiones Carboxylic Acidseeshwar saagarNo ratings yet
- Between Aldehyde and Ketones Which One Is Confirmed Using Fehling's Solution.. 1Document7 pagesBetween Aldehyde and Ketones Which One Is Confirmed Using Fehling's Solution.. 1Deva RajNo ratings yet
- Expt5 Aldol Condensation W15Document10 pagesExpt5 Aldol Condensation W15johnNo ratings yet
- FALLSEM2021-22 BCHY101P LO VL2021220106628 Reference Material II 08-10-2021 2. Kinetics of ADocument13 pagesFALLSEM2021-22 BCHY101P LO VL2021220106628 Reference Material II 08-10-2021 2. Kinetics of AHarsh AgarwalNo ratings yet
- Problem Set McMurryDocument13 pagesProblem Set McMurrypolinaNo ratings yet
- Nucleophilic Acyl SubstitutionDocument4 pagesNucleophilic Acyl SubstitutionseryuyuyuNo ratings yet
- Unit 5 Practical 3 - Calculating KCDocument3 pagesUnit 5 Practical 3 - Calculating KCMuaaz IqbalNo ratings yet
- Equilibrim ConstantDocument5 pagesEquilibrim ConstantArchibald MiguelNo ratings yet
- Aldol Condensation Reaction PDFDocument6 pagesAldol Condensation Reaction PDFaizatNo ratings yet
- Carboxylic Acid and DerivativesDocument12 pagesCarboxylic Acid and DerivativesJohn Henrick G. Uy50% (2)
- Carbonyl Compounds: SEM-3, CC-7 Problems: Assignment 1Document7 pagesCarbonyl Compounds: SEM-3, CC-7 Problems: Assignment 1Pedro SilvaNo ratings yet
- معايرة حامض الهيدروكلوريك انكليزيDocument17 pagesمعايرة حامض الهيدروكلوريك انكليزيأحمد غالب مهدي - مسائي C-1No ratings yet
- Experiment 4 Aldehydes and Ketones: Preparation and Qualitative AnalysisDocument10 pagesExperiment 4 Aldehydes and Ketones: Preparation and Qualitative AnalysisRom PeDrazaNo ratings yet
- Reactions of Alcohols, Reactions of Acids and EsterificationDocument11 pagesReactions of Alcohols, Reactions of Acids and EsterificationAlyssa PhillipsNo ratings yet
- Experiment 3 - Carboxylic Acid and DerivativesDocument3 pagesExperiment 3 - Carboxylic Acid and DerivativesFaris SyahmiNo ratings yet
- IGCSE Chemistry NotesDocument33 pagesIGCSE Chemistry NotesMay Myat ThuNo ratings yet
- Exp01 FischerEsterification ManualDocument3 pagesExp01 FischerEsterification ManualJimmy AxeNo ratings yet
- Chemistry Form FiveDocument23 pagesChemistry Form FiveNorazlin Ujang100% (2)
- MethylbenzoateDocument6 pagesMethylbenzoateLindelwa MthembuNo ratings yet
- Exp 18Document8 pagesExp 18nicolef_20No ratings yet
- Formal Report: Nucleophilic Acyl Subtitution: The Synthesis of EthersDocument3 pagesFormal Report: Nucleophilic Acyl Subtitution: The Synthesis of EthersJuris Marie G. GarciaNo ratings yet
- 8-Essence of Esterification Sp16Document7 pages8-Essence of Esterification Sp16kerredaiNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Practice Makes Perfect in Chemistry: Organic ChemistryFrom EverandPractice Makes Perfect in Chemistry: Organic ChemistryRating: 3 out of 5 stars3/5 (1)
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Catalytic Asymmetric SynthesisFrom EverandCatalytic Asymmetric SynthesisIwao OjimaNo ratings yet
- Summary of Rheology - Physical Pharmacy 2Document29 pagesSummary of Rheology - Physical Pharmacy 2Noor FarhanNo ratings yet
- Lecture 4Document15 pagesLecture 4Noor FarhanNo ratings yet
- Urrinary Tract Infection: Dania A. Kamal Helin Haider Khidir Abeer Zuheyr Jalal Physiology - PHAR 202 Spring SemesterDocument8 pagesUrrinary Tract Infection: Dania A. Kamal Helin Haider Khidir Abeer Zuheyr Jalal Physiology - PHAR 202 Spring SemesterNoor FarhanNo ratings yet
- Week 5 UltrasoundDocument26 pagesWeek 5 UltrasoundNoor FarhanNo ratings yet
- WameedMUCLecture 2021 92721835Document34 pagesWameedMUCLecture 2021 92721835Noor FarhanNo ratings yet
- Lab 3Document20 pagesLab 3Noor FarhanNo ratings yet
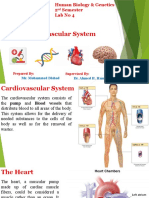
- Human Bio Lab 4 CardioDocument11 pagesHuman Bio Lab 4 CardioNoor FarhanNo ratings yet
- 1 Holdren Martin 2022 PHDDocument246 pages1 Holdren Martin 2022 PHDNoor FarhanNo ratings yet
- Principles of Pharmaceutical CalculationsDocument28 pagesPrinciples of Pharmaceutical CalculationsNoor FarhanNo ratings yet
- 25-3-2023 1st L. Analytical Chem. (Dr. Hazha)Document26 pages25-3-2023 1st L. Analytical Chem. (Dr. Hazha)Noor FarhanNo ratings yet
- Lab 1 Human BiologyDocument34 pagesLab 1 Human BiologyNoor FarhanNo ratings yet
- Pharmacy, Medical Terminology, BA Week 5. 2Document27 pagesPharmacy, Medical Terminology, BA Week 5. 2Noor FarhanNo ratings yet
- French Polishing 1Document2 pagesFrench Polishing 1tfahrenkNo ratings yet
- Developing Bioplastics in Transgenic PlantsDocument18 pagesDeveloping Bioplastics in Transgenic PlantsfreedomoneNo ratings yet
- Properties of Ointment BasesDocument1 pageProperties of Ointment BasesGisselle MuñozNo ratings yet
- Journal Pre-Proof: Materials Chemistry and PhysicsDocument25 pagesJournal Pre-Proof: Materials Chemistry and Physicshakim nasirNo ratings yet
- A Study On Mechanical Properties of Polydimethylsiloxane (PDMS) CompositesDocument13 pagesA Study On Mechanical Properties of Polydimethylsiloxane (PDMS) Compositesacademic researchNo ratings yet
- Chemistry and Reactions of Cellulose PDFDocument5 pagesChemistry and Reactions of Cellulose PDFAditya ShrivastavaNo ratings yet
- T3 Annulars BOP Operators Manual 7022 PDFDocument28 pagesT3 Annulars BOP Operators Manual 7022 PDFJohn Jairo Simanca100% (2)
- How Lyocell Is Made - Material, Manufacture, Making, Used, Processing, Steps, Industry, MachineDocument5 pagesHow Lyocell Is Made - Material, Manufacture, Making, Used, Processing, Steps, Industry, MachineFabio FotografNo ratings yet
- Tumble Dryer Mesin Pengering: Instruction Booklet Buku PetunjukDocument16 pagesTumble Dryer Mesin Pengering: Instruction Booklet Buku PetunjukMuhamad Kadriee सुंदरNo ratings yet
- One of Their Most Famous Products The Abnormal Beauty CompanyDocument7 pagesOne of Their Most Famous Products The Abnormal Beauty CompanyHuyen Trang NguyenNo ratings yet
- SIMONA901 ECTFE 901 Data SheetDocument2 pagesSIMONA901 ECTFE 901 Data Sheetdavid leeNo ratings yet
- Sodium Alginate: The Wonder Polymer For Controlled Drug DeliveryDocument10 pagesSodium Alginate: The Wonder Polymer For Controlled Drug DeliveryAinii BasriNo ratings yet
- Membrane ProcessesDocument24 pagesMembrane ProcessesMegaa Nurrahma DewieNo ratings yet
- Porous Materials For Oil Spill Cleanup: A Review of Synthesis and Absorbing PropertiesDocument12 pagesPorous Materials For Oil Spill Cleanup: A Review of Synthesis and Absorbing PropertiesAJAY KUMAR MAHAKUDNo ratings yet
- Iso 11193 1 2020Document9 pagesIso 11193 1 2020Katerin Martínez100% (1)
- BindersDocument4 pagesBindersSariyyaHeydarovaNo ratings yet
- 2019 12 KratonDocument41 pages2019 12 KratonEason HuangNo ratings yet
- Yadr I ClassesDocument20 pagesYadr I ClassesRiya JaiswalNo ratings yet
- Chemical Biology PresentationDocument26 pagesChemical Biology PresentationGourav DasNo ratings yet
- Molykote Food Grade Lubricants BROCH - EN (80-3189-01)Document6 pagesMolykote Food Grade Lubricants BROCH - EN (80-3189-01)DiogoNo ratings yet
- Effect of Carbon Molecular Sieve Sizing With Poly (Vinyl Pyrrolidone) K-15 On Carbon Molecular Sieve-Polysulfone Mixed Matrix Membrane-MainDocument9 pagesEffect of Carbon Molecular Sieve Sizing With Poly (Vinyl Pyrrolidone) K-15 On Carbon Molecular Sieve-Polysulfone Mixed Matrix Membrane-Mainyuva1611No ratings yet
- Development of Glass/Banana Fibers Reinforced Epoxy CompositeDocument6 pagesDevelopment of Glass/Banana Fibers Reinforced Epoxy CompositeNeymarNo ratings yet
- Waterproofing of Concrete FoundationsDocument37 pagesWaterproofing of Concrete FoundationsAzhar Abdul RazakNo ratings yet
- 11.carboxylic Acids - DerivativeDocument27 pages11.carboxylic Acids - DerivativeP. E. I. AcademicsNo ratings yet
- Gate 1991-2000 Textile PapersDocument69 pagesGate 1991-2000 Textile Papersmohit100% (1)
- Specialist Project Leathercraft Manual Justin Schlichter Dec 2007Document14 pagesSpecialist Project Leathercraft Manual Justin Schlichter Dec 2007dendron60% (10)
- MCS 640 PP Nonwovens - FabricDocument8 pagesMCS 640 PP Nonwovens - Fabricpaddy259No ratings yet
- Unit 1-Chapter 1. Introduction To Engg. MaterialsDocument76 pagesUnit 1-Chapter 1. Introduction To Engg. Materialssainath reddy kesam reddyNo ratings yet
- Rubber Chemical Resistance Chart V001MAR17Document27 pagesRubber Chemical Resistance Chart V001MAR17Deepak patilNo ratings yet