Professional Documents
Culture Documents
What Are The Different Places To Use The
What Are The Different Places To Use The
Uploaded by
structuredes.1Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
What Are The Different Places To Use The
What Are The Different Places To Use The
Uploaded by
structuredes.1Copyright:
Available Formats
Open in App Sign In
What are the different places to use
the different values of R - gas
constant (like, where to use 8.314
JK⁻¹mol⁻¹ and where 0.082
LatmK⁻¹mol⁻¹)?
All related (34) Recommended
Assistant
Bot · May 2
The gas constant, denoted by 40 R 41 , can
be expressed in different units depending on the
context and the units of other quantities involved in
the calculation. The two common values for the gas
constant are:
1. 40 R = 8.314 \, \text{J K}^{-1}
\text{mol}^{-1} 41 - This is the gas
constant expressed in joules per kelvin per
mole. It is commonly used in calculations
involving the Ideal Gas Law when pressure
is in pascals, volume is in cubic meters,
temperature is in kelvin, and the number of
moles is in moles.
2. 40 R = 0.082 \, \text{L atm K}^{-1}
\text{mol}^{-1} 41 - This is the gas
const Continue Reading
Upvote
Jake Dickerman
HS Chemistry and Physics Teacher · Author h… · 4y
1.3K answers and 2M answer views
All of the values of the gas constant - R - represent
the same QUANTITY. Where they are different is in
their UNITS. What you want to do when picking out
a value to represent R is see what units R needs to
cancel. If your pressure is measured in atmospheres
and your volume in liters, you need to make sure
that R is in terms of atmosphere Liter per mol kelvin
(~.0821). If your pressure is measured in Torr and
your volume in meters cubed you need to make sure
you’re using Torr m^3 per mol kelvin (~.624). If you
are measuring a gas in terms
Continue of the energy present
Reading
and you’re measuring the energy in
Upvote · 1
Sponsored by Berlin School of Business & Innovation
Receive guidance on admissions & visa for
your master's degree.
Earn your BSBI master's degree in strategic
marketing, logistics or Global MBA in Barcelona.
Learn More
20
Related questions (More answers below)
What are some values of R (gas constant)
important for the JEE level?
121,840 Views
How do I decide whether to use 0.0821 or
8.314 for R?
170,540 Views
What is the difference between 1 kgf and 1
Newton?
68,535 Views
How do I convert v/v to w/w and what does it
mean?
52,002 Views
What is the value of R (gas constant) in
different units?
119,804 Views
Piyush Kumar
Studied at Institute of Science, Bana… · Updated 5y
s Hindu University (Graduated 2022) ·
Whatby
RelatedUpvoted are someChowdhury,
Abhrajit values of R (gas
constant)
99.78important forEntrance
percentile Joint the JEE level?
Examination
Generally 3 valuesMain,
of RJoint Entrance
is used
Examination (2024)Author has 124 an
Wait…swers and 242.7K answer views
i have better values for you
1. 1/12 Lt-atm/K-Mol
2. 25/3 joule/K-Mol
3. 2 calorie/K-
Upvote · 396 17 2
Ted Horton
Over 25 years experience teaching Physics. · … · 4y
uthor has 401 answers and 574.7K answer vie
Edit: After
ws
I wrote this I realized the question poster
did not ask about using Boltzmann’s constant.
However, I want to leave this answer posted. If I get
too many negative comments I will remove the
answer.
Other answers written here are good and remind
you to always look at how the units in your
calculation work out. The units you use for the gas
constant should be consistent with the other units
in your calculation. However, there is one more
important difference when using R vs kB in the
ideal gas law. The law can be written two different
ways with the constants:
Continue Reading
PV =
Upvote
Taresh Batra
Studied Electrical and Electronics Engineerin… · 6y
at BITS Pilani, K. K. Birla Goa Campus (Graduat
RelatededCan
2023)we use value 2 for gas constant R
rather than 8.314 kJ mol K?
It depends on the units.
If you are expressing work done in terms of calories
then you can use 2 Cal/mol K but if in joules, then …
8.314 J/mol K
2 used here, is nothing but an approximate value of
8.314/4.184
So, it comes from the relationship between J and
Cal.
And please , R is not equal to 8.314 K J/mol K
Upvote · 47 3
Sponsored by Cyclr
Want a faster way to provide your SaaS users
with integrations?
Cyclr's low-code embedded integration platform
will help you scale your in-app integrations in no
time.
Free Trial
Related questions (More answers below)
Can we use value 2 for gas constant R rather
than 8.314 kJ mol K?
30,848 Views
What is a zero order, first order, second order,
and third order reaction?
4,949 Views
How do I convert v/v% to w/w%? I want to
make .3%(v/v) HCl for 50 ml solution, but the
HCl is 33%(w/w) pure.
8,233 Views
What value of R gas constant should be used?
50,405 Views
When do we use the value of universal gas
constant as 0.0821 and 8.314?
12,621 Views
Mike Jones
MAEd in Chemistry & Physics, Western Caroli… · 4y
a University (Graduated 1974) · Author has 6.2
In an ideal
RelatedK answers gas answer
and 7.2M law problem,
views how do
you know which value of gas constant R to
use? I've looked at the tables but I don't quite
understand what the units have to do with the
R-value.
The gas constant, R, can be written with a number
of different units, usually the pressure unit. Pick the
value of R based on the pressure units in the
problem or the pressure units desired for the
answer.
R = 0.08206 Latm/molK
R = 0.08314 Lbar/molK
R = 8.314 LkPa/molK
R = 62.36 Ltorr/molK
For instance, consider 1 mol of an ideal gas at
standard temperature and pressure (1 atm and
273K). At this temperature and pressure 1 mol has a
volume of 22.414L.
PV = nRT
R = PV / nT = 1.000 atm x 22.414L / 1 mol / 273.15K
= 0.08206 Latm/molK
Continue
This gives us a starting Reading
point for R, then the
different values of
Upvote · 7 2
Michael Mombourquette
Retired Chemistry Prof, Church member, Knig… · 5y
of Columbus, · Author has 6.2K answers and 1
Which
Related5.7M R (universal
answer views gas constant)
should we use when converting from Kc to Kp
or from Kp to Kc (0.082 or 8.314)?
Use whichever one you need to cancel the units of
pressure and get units of concentration.
This is only done if the system at question is a gas
phase reaction. The concentration of a gas phase
reaction is n/V. the pressure is P. they are related in
the ideal gas equation.
PV=nRT. if you are using P and V in atmospheres
and litres, respectively then you should use R in
those units too. 0.08203 L-atm/mol-K to properly
cancel out the units.
Thus, P = n/V * RT is the conversion equation
between C and P.
If your pressure and volume is using SI units then
use R = 8.3145 J/molK as these are SI units.
Continue Reading
Pers
Upvote · 5
Sponsored by Atlassian
See what you can learn today—and uncover
where you might go tmr!
Unlock your potential with free learning at Atlassian
University. Get skills, earn credentials, for free!
Sign Up
867
SHIVAM SAHIL
Physics lover, mathematics lover, fictional sci… · 6y
ce lover spiderman fan · Author has 388 answe
Relatedrs What value
and 606.7K of R gas
answer viewsconstant should
be used?
Depending upon the way of solving, If you deal a
problem with ideal gas equation in terms of moles
then the value is 8.314 J / mol. K.
whereas in engineering thermodynamics, many of
its problems are associated with mass equations
hence, there R varies for every gas, for air its
approximately
287.058 J /kg-K. Also depending upon the pressure,
volume units etc, its value changes as well.
These units are as follows:
8.3145 L kPa / mol·K 1 atm = 101.32 kPa
= 8.3145 J / mol·K 1 J = 1 L kPa
= 1.987 cal / mol·K 1 cal = 4.182 J
= 62.364 L torr/ mol·K 1 atm = 760 torr
Thanks!
Upvote · 11
Biswajit Ghosh (!"#!$% &'()) (िवश्विजत घोष)
Bong-guy||Civil Engineer||Tourer||Photograph… · 5y
|Song-lover · Author has 147 answers and 600.
Related2KWhat
answerisviews
the value of R (gas constant)
in different units?
Thanks for the question.
The different values of gas constant ‘R’ are as
follows,
1. 8.314 J/K/mol
2. 62.364 L Torr/K/mol,
3. 8.314 x 10^-2 L bar/K/mol,
4. 8.314 Pa m^3/K/mol,
5. 8.20578 x 10-2 L atm/K/mol,
6. 1.98722 cal/K/mol.
Thanks.
Upvote · 28 4
Sponsored by Betterbuck
Was sind die dümmsten Dinge, für die
Menschen zu viel ausgeben?
Hier sind die 5 schlimmsten Dinge, für die
Menschen zu viel ausgeben.
Learn More
17
Rhik Banerjee
BTech in Mechanical Engineering, Manipal Ins… · 5y
ute Of Technology (MIT) (Graduated 2024)
Related What is the value of R (gas constant)
in different units?
Value of R (gas constant) has many values
depending on the unit system:
Generally by Ideal Gas Equation
R=PV/nT where P is pressure, V is volume, n is the
number of moles of gas, T is temperature. (let this
be equation 1)
At S.T.P (Standard Temperature and Pressure)
P= 1 atm, T=273K (kelvin) or 0° Celsius, taking n as
1 and so the volume of gas is 22.4 lit (gram molar
volume), by calculations in equation 1, R=0.0821
lit.atm/mol.kel
In C.G.S system, n and T remains same(no unit
change), P=1 atm = 10^5×101325 dyn/m^2, V=
22400ml, then by equation 1, R=8.314×10^7
ergs/Mol. kel Continue Reading
In M.K.S system
Upvote · 97 P=101325N 8 1
Pralhad Shenoy
B.Sc. in Chemistry, University of Mumbai (Gra… · 6y
uated 2021) · Author has 62 answers and 136.
Related3KWhen
answerdo we use the value of universal
views
gas constant as 0.0821 and 8.314?
Whenever the unit for volume is in litres and the unit
for pressure is in atmosphere you need to use
0.08214. If the units of volume and pressure are in
any multiples of the SI unit then convert then
convert them into the standard form of that unit and
use 8.314.
Upvote · 10
Purna Chandra Sahu
M. Sc. in Pure Chemistry & Inorganic Chemist… · 3y
, Calcutta Uniiversity (Graduated 1987) · Author
RelatedhasHow
2.9Kdo we know
answers and 5M when toviews
answer use R=8,31
or R=0,082 in chemistry?
When we use unit of pressure as Newton /m^2 and
volume in m^3, we have to take R = 8.314
Jmol^-1K^-1 and if we use unit of pressure in atm
and volume in L, we have to use R = 0.082
Lmol^-1K^-1.
Hope, this helps.
Upvote · 5
Adarsh Kumar
B.A from University of Delhi (Graduated 2022) · 4y
Related Can we use value 2 for gas constant R
rather than 8.314 kJ mol K?
First thing is that,
R= 8.314 J/K/mole.
Now coming to your question yes we can use this
value of R but when the equation requires it.
R= 1.985 calorie per degree celsius i.e. when we
have to find answer in calories then we can use
R= 2 cal/deg celsius/mole(approx).
Upvote · 1
Related questions
What is x2 − x2 ?
61,000 Views
What is the meaning of w/v and w/w in
chemistry?
92,579 Views
How do I decide which value of r (gas
constant) to be used?
1,576 Views
In an ideal gas law problem, how do you know
which value of gas constant R to use? I've
looked at the tables but I don't quite
understand what the units have to do with the
R-value.
11,307 Views
What is the relation between Newton, Joule,
and Metre?
8,365 Views
How do you calculate the methanol ppm from
v/v%?
5,211 Views
What will be the unit of pressure if R is 8.314
and t in Kelvin and V in liter for ideal gas?
12,231 Views
What is the unit of a gas constant?
5,856 Views
How do I convert WT% to volume?
1,349 Views
Magnesium metal reacts with hydrochloric
acid to produce hydrogen gas: Mg (s) + HCl
(aq) → H2 (g) + MgCl2 (aq) If 2.30 g of Mg is
reacted with an excess amount of HCl at
SATP, what volume of hydrogen gas will be
produced?
5,660 Views
What is the value of R, the gas constant, used
in the ideal gas equation, PV = nRT, if P is in
kPa, V is in dm³, T is in K and n is the number
of moles? What are the units of R?
1,876 Views
What is the difference between the universal
gas constant and a gas constant 'R'?
19,845 Views
How do I convert wt% to mol%?
33,477 Views
How can you prepare 10 ml of 100%, 80%,
60% 40% and 20% ethanol from 95%
ethanol, in water?
59,084 Views
What is the value of R for one mole of a gas
under an STP condition?
9,436 Views
About · Careers · Privacy · Terms · Contact · Languages ·
Your Ad Choices · Press · © Quora, Inc. 2024
You might also like
- Nidek ARK 730 Autorefractometer Service Manual PDFDocument83 pagesNidek ARK 730 Autorefractometer Service Manual PDFPetrica DascaluNo ratings yet
- Ideal GasDocument12 pagesIdeal GasJasminSutkovicNo ratings yet
- Lab Manual For Various Experiments in Chemical EngineeringDocument151 pagesLab Manual For Various Experiments in Chemical EngineeringAmey Pathak83% (6)
- IEE Wiring RegulationsDocument64 pagesIEE Wiring Regulationshaiob50% (2)
- Universal Gas Constant Values in DiffereDocument1 pageUniversal Gas Constant Values in Differestructuredes.1No ratings yet
- Value Constant Formula: DetailedDocument1 pageValue Constant Formula: Detailedstructuredes.1No ratings yet
- Value of Universal Gas Constant: FormulaDocument2 pagesValue of Universal Gas Constant: Formulastructuredes.1No ratings yet
- November 2013 ChE Board Exam QuestionsDocument3 pagesNovember 2013 ChE Board Exam QuestionsJayson Ordinaria100% (1)
- CRE3 Fogler 3 Rate Laws 1Document72 pagesCRE3 Fogler 3 Rate Laws 1DeneshVijayNo ratings yet
- Ideal Gas LawDocument4 pagesIdeal Gas LawAbdul RaufNo ratings yet
- Tricks & Formulas of Chemistry-I (NUST+NUMS) PDFDocument57 pagesTricks & Formulas of Chemistry-I (NUST+NUMS) PDFHamair Ali100% (1)
- A2 Tick ListsDocument21 pagesA2 Tick ListsGirish AdapaNo ratings yet
- Environmental Chemistry ReviewDocument8 pagesEnvironmental Chemistry ReviewCarlos CabrejosNo ratings yet
- Introduction & Overview To Chemical Reaction Engineering IIDocument12 pagesIntroduction & Overview To Chemical Reaction Engineering IIshubhamNo ratings yet
- CRE - II Unit 2 First PartDocument16 pagesCRE - II Unit 2 First PartTomble BravoNo ratings yet
- Chemical EquilDocument11 pagesChemical EquilAditya VermaNo ratings yet
- 12chemistry Eng 201415Document247 pages12chemistry Eng 201415Vaibhav RohillaNo ratings yet
- CREII-Module-I - Lecture 1Document38 pagesCREII-Module-I - Lecture 1Aditya parasNo ratings yet
- Properties of Gases - 220308 - 154934Document28 pagesProperties of Gases - 220308 - 154934Dhruvi PadmaniNo ratings yet
- 5 Concept of ChemistryDocument8 pages5 Concept of ChemistrySourabh ChoudharyNo ratings yet
- Chemical Reaction EngineeringDocument93 pagesChemical Reaction EngineeringGuru Raj BhattNo ratings yet
- Reactor Engineering - Introduction: PreprintDocument21 pagesReactor Engineering - Introduction: PreprintRitesh ChauhanNo ratings yet
- Thermodynamics For Beginners - Chapter 5 WORKING WITH IDEAL GASDocument35 pagesThermodynamics For Beginners - Chapter 5 WORKING WITH IDEAL GASDudu MamanNo ratings yet
- Chemical Equilibrium-UAPDocument26 pagesChemical Equilibrium-UAPMd. Mujahid HasanNo ratings yet
- Lesson Plan 3Document8 pagesLesson Plan 3sakhi dewaniNo ratings yet
- Ideal Gas Law NotesDocument4 pagesIdeal Gas Law NotesPrincess Jean GalabinNo ratings yet
- Reactor Design With Matlab in A Manufacturing EnvironmentDocument11 pagesReactor Design With Matlab in A Manufacturing Environmentமுத்துக்குமார் சிவகாமி0% (1)
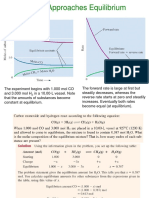
- The Relationship Between KC ND KPDocument15 pagesThe Relationship Between KC ND KPamroceamroceNo ratings yet
- EquilibriumDocument40 pagesEquilibriumAndreaMarkhamNo ratings yet
- LAS 9 Gen Chem Ideal Gas LawsDocument4 pagesLAS 9 Gen Chem Ideal Gas LawsJorgia lianne UrbanoNo ratings yet
- Equlibrium 2022-1Document15 pagesEqulibrium 2022-1Huzaifa Ahmed FarooqiNo ratings yet
- Ideal Gas LawDocument10 pagesIdeal Gas Lawchaotictrust28No ratings yet
- Seminar 28Document31 pagesSeminar 28Sunil PillaiNo ratings yet
- Reactor DesignDocument39 pagesReactor DesignSteila Marris Bolhano0% (1)
- Important Points of Chemistry For Entry Tests (Part 1 & 2)Document115 pagesImportant Points of Chemistry For Entry Tests (Part 1 & 2)waji ul hassanNo ratings yet
- موازنة الطاقة الثالثةDocument32 pagesموازنة الطاقة الثالثةكرار عبدالحسين قاسم100% (1)
- Ideal GasDocument12 pagesIdeal GassteveislaryNo ratings yet
- Lecture 7 Chemical EquilibriumDocument54 pagesLecture 7 Chemical EquilibriumHiep NguyenNo ratings yet
- Lecture 9 - Chemical Equilibrium and Solution ChemistryDocument7 pagesLecture 9 - Chemical Equilibrium and Solution ChemistryDeresse MogeseNo ratings yet
- CH E 345: Lecture 3 (Arrhenius-Equation)Document9 pagesCH E 345: Lecture 3 (Arrhenius-Equation)nmoverleyNo ratings yet
- Chemical Thermodynamics: by Jorge Omar Gil Posada CH9: An Introduction To MixturesDocument77 pagesChemical Thermodynamics: by Jorge Omar Gil Posada CH9: An Introduction To MixturesCarolina FernandezNo ratings yet
- Chapter 6Document17 pagesChapter 6helloblarg100% (3)
- Chemical Kinetics CH 290Document263 pagesChemical Kinetics CH 290jastin michaelNo ratings yet
- CREII-Module-I - Lecture 1 PDFDocument36 pagesCREII-Module-I - Lecture 1 PDFshubhamNo ratings yet
- Chemical Kinetics: Prentice Hall © 2003Document59 pagesChemical Kinetics: Prentice Hall © 2003ZafirahAhmadFauziNo ratings yet
- 03 Units and Conversion StudentsDocument21 pages03 Units and Conversion StudentsNur AmaninaNo ratings yet
- Main ISM Ch07Document14 pagesMain ISM Ch07Shoja Sammy RahimianNo ratings yet
- 9.3 Ideal GasDocument53 pages9.3 Ideal GasshahoodaNo ratings yet
- 1.1 How FastDocument14 pages1.1 How FastG M Ali KawsarNo ratings yet
- PVT (Properties of Petroleum Fluids)Document32 pagesPVT (Properties of Petroleum Fluids)Oscar Mauricio TellezNo ratings yet
- Chapter 3 Rates Law and StoichiometryDocument60 pagesChapter 3 Rates Law and StoichiometryMalek Marry AnneNo ratings yet
- Methanol SynthesisDocument14 pagesMethanol SynthesisRahmad Fajar TanjungNo ratings yet
- 4.1 Ideal GasesDocument22 pages4.1 Ideal GasesAnonymous o97HYLpe0No ratings yet
- Topic 05 - States of Matter - TutorsDocument17 pagesTopic 05 - States of Matter - TutorsTran Nhat ThangNo ratings yet
- 24 - ALE 24student Key Complete - Ideal - Real Gases-Kin Mol Theor-Compre Qs - F2008Document4 pages24 - ALE 24student Key Complete - Ideal - Real Gases-Kin Mol Theor-Compre Qs - F2008Sheyla PavajeauNo ratings yet
- Lecture 12 Principle Cal 2XEDocument34 pagesLecture 12 Principle Cal 2XEqwerty fkvorkcjdkNo ratings yet
- Chapter 4 Reactor DesignDocument48 pagesChapter 4 Reactor DesignAhmadNo ratings yet
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarFrom EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarNo ratings yet
- Physical and Chemical Equilibrium for Chemical EngineersFrom EverandPhysical and Chemical Equilibrium for Chemical EngineersRating: 5 out of 5 stars5/5 (1)
- 01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-14Document2 pages01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-14structuredes.1No ratings yet
- Kinetic Theory of Gases - Chemistry LibrDocument1 pageKinetic Theory of Gases - Chemistry Librstructuredes.1No ratings yet
- 202 Heat-Exchanger-DesignDocument74 pages202 Heat-Exchanger-Designstructuredes.1No ratings yet
- Economics of Gas Gathering Cashflow of TechsimDocument77 pagesEconomics of Gas Gathering Cashflow of Techsimstructuredes.1No ratings yet
- 01 - Getting To Know Lightweight Design - Rifa - SoSe2024-part-5Document3 pages01 - Getting To Know Lightweight Design - Rifa - SoSe2024-part-5structuredes.1No ratings yet
- 01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-2Document5 pages01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-2structuredes.1No ratings yet
- ChicagoSchoolOfData FormFillsDocument63 pagesChicagoSchoolOfData FormFillsstructuredes.1No ratings yet
- 01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-3Document3 pages01 - Getting To Know Lightweight Design - Rifa - SoSe2024-Part-3structuredes.1No ratings yet
- Excel References Soot and Pahs CalculationDocument2 pagesExcel References Soot and Pahs Calculationstructuredes.1No ratings yet
- Advanced MRTLS Class 1 Lecture Notes-1Document2 pagesAdvanced MRTLS Class 1 Lecture Notes-1structuredes.1No ratings yet
- Case Study Chemical-Engineering-Design2021 Purchased Equipment CostsDocument5 pagesCase Study Chemical-Engineering-Design2021 Purchased Equipment Costsstructuredes.1No ratings yet
- Chemical Inventory FormDocument12 pagesChemical Inventory Formstructuredes.1No ratings yet
- Chemistry Assignment1Document2 pagesChemistry Assignment1structuredes.1No ratings yet
- Chem Practice Paper 5 QPDocument10 pagesChem Practice Paper 5 QPSANAJ BSNo ratings yet
- Electronic Noises Interface Circuits and OscillatorsDocument21 pagesElectronic Noises Interface Circuits and Oscillatorslucaplat11No ratings yet
- Pricelist: Lugs & GlandsDocument18 pagesPricelist: Lugs & GlandsabhishitewariNo ratings yet
- NP Sto 50 Silicone Transformer Oil ClearcoDocument2 pagesNP Sto 50 Silicone Transformer Oil ClearcoAkshay MutalikNo ratings yet
- Direct Shear Test On Cohesionless SoilDocument10 pagesDirect Shear Test On Cohesionless Soilsamantha richardsonNo ratings yet
- ST1803DHI: High Voltage Fast-Switching NPN Power TransistorDocument6 pagesST1803DHI: High Voltage Fast-Switching NPN Power TransistoredgardoNo ratings yet
- Trigonometry - Primary Trigonometric Ratios - Lengths All PDFDocument12 pagesTrigonometry - Primary Trigonometric Ratios - Lengths All PDFElaine BatistaNo ratings yet
- Ansys Dynamic AnalysisDocument24 pagesAnsys Dynamic AnalysisAll in one EthiopiaNo ratings yet
- Q Manager System-Poush HygieneDocument2 pagesQ Manager System-Poush Hygienebharathirajan964No ratings yet
- An Introduction To ElectronicsDocument253 pagesAn Introduction To ElectronicsAmal KrishnanNo ratings yet
- Chemistry Paper 1 2022 HigherDocument32 pagesChemistry Paper 1 2022 HigherJohn RodNo ratings yet
- Modular Lithium-Ion Energy Storage SystemDocument2 pagesModular Lithium-Ion Energy Storage SystemNatália DominguesNo ratings yet
- Sepharose Fast Flow Ion Ex ChangersDocument5 pagesSepharose Fast Flow Ion Ex ChangersTran TuanNo ratings yet
- IRC 112-2019 - Shear ClauseDocument1 pageIRC 112-2019 - Shear ClauseSekharSangadiNo ratings yet
- Dynamics SolDocument55 pagesDynamics SolJayson PagalNo ratings yet
- F5 2 TekananDocument176 pagesF5 2 TekananAZRI AZMI BIN ABD AZIZ MoeNo ratings yet
- One-Antenna Radiation Pattern Measurement of On-Wafer Antennas in Probe Station EnvironmentDocument9 pagesOne-Antenna Radiation Pattern Measurement of On-Wafer Antennas in Probe Station EnvironmentPMMNo ratings yet
- Curso NeutrinoDocument45 pagesCurso NeutrinoJuan ZapataNo ratings yet
- Cornish 1907 The Jamaica EarthquakeDocument36 pagesCornish 1907 The Jamaica EarthquakeDrift KingNo ratings yet
- 1.1 Course ContentDocument6 pages1.1 Course ContentAhmad HararaNo ratings yet
- Math p2 g11 QP Nov2020 EnglishDocument10 pagesMath p2 g11 QP Nov2020 EnglishlwandilemnyangoNo ratings yet
- Mechanical BehaviourDocument12 pagesMechanical BehaviourJaouad OuaâzizNo ratings yet
- Alternator Stators - Construction and Design: Commercial Applications & Electrical ProjectsDocument4 pagesAlternator Stators - Construction and Design: Commercial Applications & Electrical ProjectsEZEJI GEORGENo ratings yet
- BSB-310-Output Module-MonitoredDocument2 pagesBSB-310-Output Module-MonitoredIrlan LeiteNo ratings yet
- Oxford MIG Welding Machines: MIGMAKER Range 180 Amp To 330 Amp Single & Three Phase ModelsDocument6 pagesOxford MIG Welding Machines: MIGMAKER Range 180 Amp To 330 Amp Single & Three Phase ModelsMariusz NocuńNo ratings yet
- Partner Price List: Effective Monday 14th October 2019Document17 pagesPartner Price List: Effective Monday 14th October 2019Noel JagoNo ratings yet
- Created by C. Mani, Deputy Commissioner, KVS RO GurgaonDocument28 pagesCreated by C. Mani, Deputy Commissioner, KVS RO Gurgaonjaindevansh0% (1)