Professional Documents
Culture Documents
Usp
Usp
Uploaded by
terminatrix0 ratings0% found this document useful (0 votes)
4 views8 pagesusp
Original Title
usp
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentusp
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
4 views8 pagesUsp
Usp
Uploaded by
terminatrixusp
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 8
usp 40 Physical Tests # (671) Containers—Performance Testing 1
(671) CONTAINERS—PERFORMANCE TESTING
INTRODUCTION
{e's the purpose of tis chapter toprovide standards forthe functional properties of packaging systems used for solid oral
dosage forms (S00F) and fiqud ool dosage forms (LODF) for pharmaceuticals and deta supplements. Detintlons tht apaly
to ths chepter are provided in Packaging on Storage tequireenents (659). The tests that follow ere provided to determine the
‘pelsture vapor tranerission rate (water vapor permeation rate) and spectral transmission of plastic cortaines,
ett methods are provided to measure moisture vapo: transmission rates th may bs usehl for phrmaceutical and dietary
‘upplement manufacturers to determine the level of barrier protection provided by packaging systems for SODK, Adticeal
imethdds are provided to determine cassication of packaging ystems for SODF and LODF that ae repackaged by organza
tianzor wien being elspensedl on prescription by prmacisassngle-cinit and enlipe-unit containers (Tole T}-There ony
bbs acklitonal packaging systems where the test methods in tls sectin could be applied; however. ary deviation should be
scribed. If other methods are used ta measure moisture vapor transmission zate, these methods should be deserted in sel
Gent detait o justly their use.
Table 1, Motstore Vapor Transmission Test Methods for Packaging Systems
Liquid Oral
Solid Oral Dosage Forme Dosnge Forms
‘raison for Single Unit | Ciasiaton for Mule
Barat Protection Dereri. | Clasieationforsuliple- | “Containers end Uni-Dose | Unit Cantons sed tak
Section Title ilies ‘Uni Contsina onuaigets Dose Containers
Maltplsuit conte] Mulounit contains
‘wih sea icace © Drgeh- | “with lsu apted od
2 uz and gle unit or | seatintactorbreaced) | Singie-unitand unit-tose | Multiples and singlet
‘nppiteatan nit dose eonsinere sate cotainessinsedled state | ‘contacwrs
Fenuiscusers ‘Manutacturere Manufecturer
Packages ackages Packages
Fepackagees epackagers Repackagers
Users Manufacturers Promuce Pratnags Phatmecies
Change to read: Me
MOISTURE VAPOR TRANSMISSION
Barrier Protection Determination for Packaging Systems for Solid Oral Dosage Forms
‘This seclion describes moisture vapor transmission test methods fr mulliple unit containers (Method high baer single.
Soi a umiedose containe's (Method 2), and low bare single-unitand unitedose containers (Method 3) used by pharma,
‘ikea manufacturers to package SODF. The purpose of this test method i to obtain relable and speciic moistae vonor
Tstsion fates that can be used to dscriminate among brie performance of packaging systerrs used for regulates arti
les; the method is based upon ASTM method 07709."
‘This method contains the following attributes
2. Reports specific moisture Vapor transmission value fora container cather than a classification
‘8 Provides sufficient sensitivity and precision to alow difereatia
© Beiations ted fo testing these packaging systems are the same as those used for accelecated sabtiy testing of the
primary packaging of regulated articles (typically 40°/75% 2H),
EQUIPMENT
“Tho follwing items should be avatabe:
*A.balance for weighing the fest specimens that hat sufficient capac to weigh the test specimens throughout the period
olahe test Gee Weishing on an Anata! Batnce (1251). The balance shal hove sesllsey adocuate ree
Girences in welght tm one time pont to tha net. The weghing wacertanty sal be salt than SH of le wet
gain om ons time point tothe nex. The Weighing uncertainty is typealy three nes the balaneeresoktton sera
Asan example, 2 balance with a resolution of 0.1 mg isaceeptable fer paclaging systems whose weight an rr ine
interval is more than or equal to 6 mg [(0-1 x 3)/5%, which Is GO times the balance sensu
‘+A chamber copable of maintaining 40:42" and 752306 Rh
vr ee Santa Te ets fox Wenn Wate Yepor Tasso ate QW) al Panmacea aes and sen jbl by AST ete
‘onal 100 Su ber Dive .0. Sox C70, West Candolochen PA luzmao83
2 4671) Containers Performance Testing / Physical Tests
usp 40
DESICCANT,
Method 1: The desiccant is anhydious calcium chloride in granular form, Other desiécants, such-2s a molecular sieve or
fica gel may be suitable. W anhydrous calcium chloride is used, pre-dy at 213 5* for = Yoh to-ensure that any henahy=
‘rate present i fully converted tothe anhydrate. Cool the desiccant ina desiccator for atleast 2 h before use
[Methods 2 and 3: The desiccant i silica gel molded ina form tof the size and shape of tne ister cavity used Otter
deslccants may be suitable, for example, a molecular sieve. I silica gels used, pre-dry in circulating hot a coor af org of
{0 concitions: 155-£5* for 3/4 Yeh oF 150 5° for 4 & eh. a molecular seve is used, preedry in 2 mule nace or
£25 525~ Dey the 44 and 3A sieves fof 3M & Yb; diy the 13X sieve for SJ. Yel. Cool the desiccant in a desiccator fos at
feast 2 h before use, (NoTE—it has been shown? that anhydrous ealeum chloride may contain calcium hexshiydvote nivel
loses water only when the temperature reaches 200°)
PROCEDURE
Method 1: Use 15 mulipte-unit containers and 15 closures represrtativ of Ue stat to be tested, Prepare the test
specs y ling each mukiple-sunit conteiser two-thirds with desiecant theo, far sclew type osures, apply theese us
ing the tou thats within the range of ihtness specie in Table 2 For other closure types, apply eccording tothe nen
des method. Ersurethat.a proper sel hasbeen made withthe itended membrane tothe and se of te beste tne sete
‘iy each multiple-unt: container with indelible ink: Do not use Inet I there isa heed to inerense the precgon a ne meter
0, the user can tes the system without the closure as long as an immperious seal rains on the eons
desea, vig each.multiple-unit container at ambient temperature and 2 Recor this wright for Uige ze, but donot
«use it in the calculation of permeation; Place ali contaifiers ia the test chamber (40°/759% RH) within 1 h of weighing. Weigh all
trukiplsni contanes a Une intervals of 7 days +1. Weigh the mulpesunk containers t7, 14,2128, and Je davcee
‘get steady-state datapoints (The time interval from time Oto day 7 the paiod of equllbrlion) Phos to vesting ce
time interval, equibrate the containers for about 30 min a the weighing temperature and RIL initthe Une cove the
intial weight ofeach test canta (mg)
G_~ average final weight ofthe controls (mg)
Cs avernge inal weight ofthe canoe (rng)
UNore--Where the moisture vapor transmission rates measured are less than S mg/dey, and where the controls are observed
t2feacs equim within 7 days the individual molsture vapor transmission rates my be deteined more acearstly by
pan he 7-day test container and control container weights as Wand C, respectively, nthe calculation, In this case, adae
BE jeatinterval for Class 4 (see: Clesication) would be NLT 28 days flowing the inital 7-day equilibration period fe tel ot
35 days). ]
Method 2: Use this procechire for packs (e.g, punch-ovt card) that incorporate a number of separately sealed unitdose
Spataines or biters. Seal a sufelnt numberof packs, such that NLT Tour packs and a total of NLT 10 untedove coutcnon or
Sister filed! with one pele in each unitare tested. Sea -cortesponcing number of erapty pcs, cach pack containing the
‘Other methods may be emplayed to maintain these
thereof (se Clossfcator, remove the packs from the chamber, and allow them
i pauilbrate for about 45 min. Record the weights ofthe individual pack, nd retum them to.the chamber. Weigh tne eon,
Tepbie aunt and divide the total veignt by the number of control packs to obtain the average emply pack ight,
IGT any indicating pellets tun pink curing the procedure, ori the average pellet weight increase ary pack ancoeds
srametaminae the tes and régarl only ener determination as vl] Calculate, tow significant igus, the averege ake
‘of moisture vapor transmission in mga, for each untadose contains orbiter in each pack takers
DUN AN — WG = GQ)
X= umber of days expired inthe test period (beginning after (he inital 24h equilibration period)
X= number of separately sealed units per pack
W, final weight of each test pack (mg)
W, = initial weight of cach test pack (mg)
G__ final weight of the cantrol packs (ma)
&_ = intial eight ofthe control packs (rg
ane esctant stated for Method J and Method 2, the tet a. control containers or pack are weighed after evary 24
Fine a yaitable test intervals for the final weighings. Wand Cy aes flows: 24 8 For Css Dy 28 h for Class C7 days or
Gass abc NLT 28 days for Class A.
Classification: The individual unit-dose containersas tested in Method 1 are designated as follows: Class Alf nat more
Narr 1 tO containers tested exceeds 0.5 mayday in arasture vapor transmission eat and rene exceeds 1 mg/day: Cos 8 i
NMT 1 of 19 containers tested exceeds $ mg/day and nene exceeds 10 ma/day: Class cil NMP } of 10 contaner restoa
Sees Re matday and none exces 40 mg/day; ane Clas Dif the containers ested meet none of the moisture vapor tans-
mmission rate requirements.
Fg fects teed in Method 2 ace designated! 9 follows: Coss if no pac tested exceeds 0.6 malay Ii average blister
stu vapor transmission rate; Coss if no pack ested ekcaeds 5 malay in average blister mole vapor tareriesion
Tag Fs Cif no pack testec exceeds 20 mafday in average blister moisture vapor transmission rate; and Clos 04 lhe packs
tested meet none of the above average blister meisture vapor Lansmision rate tequiventonta,
Packaging System Classification for Multipie-Unit Containers-and Unit-Dose Containers for Liquid
Oral Dosage Forms
The following procedure:and classification scheme are provided to evaluate the moisture vapor transmission characteristics
mratpleurit contalers, Th information gathered should be used to make an informed! judginent regarding the suitability
OLUGe Packaging sjstem for LODE. [NoTe—Determaine the weights of inlvidualcontainer-closure systems (Bott, mer seal
Used, and closure) beth 25 tare weights and fll weights, to the nearest 0.1 mg ifthe bottle capacity less than 260 mls the
usr 40 Physical Tests ! (671) Containers—Pertormance Testing 7
‘earest mg ithe bottle capacity is 200 mL or more but les than 1000 mt; orto the nearest centigram (10 mg} if the battle
apacity 1600 mL or more}
PROCEDURE
Select 12 bots fa unitorm size and type, end clean the sealing surfaces with » inttee cloth, Fe each btle with a seal
‘tase lines Gl applicable), and closure, Number each continer-lesura system, and record the lare weight,
Remove te closures and, usiag a pipet, il 10 botles with water to the il capacity. Fl two containers wit glass bead, to
{he same-wetght as the fled test conainas. fusing screw closures, poly a orgue tat is within the range specie i Teble
2, afd store the sealed containers at temperature of 252° and a relive humdiy of 402286. Alter 356-1 h(1a Gay),
‘ecord the wright ofthe individual containers, an calculate the water weightloss rate, n percent per year, or aach bee
tokens
[ar Wi) — Cat = Wed = (Mer ~ Wen) 365 100MM, 2 WANE VAS ie ei,
tial weight of each individual bottie ()
tare weight
‘weight of each individual bottfe (9 at 14 days
iniiat weight of the control container at day 1
eight of the control container a.14 days
CLASSIFICATION
The packaging system meets the tequirements far tight if the percentage of water weightloss does not exceed 2.5% per
year in NMT1 of the 20-test containers and does not exceed 5.09% per year in any-of ther.
SPECTRAL TRANSMISSION
Apparatus:
Use a spectiophiotometer of suitable sensitivity and accuracy, adaptetor measuring the amount of light ransmited by
Plastic materials used for pharmaceutical containess In addition, the specleaphotometer is capable of measuring and fecerde
ing light transmitted in difused as wel 3s parallel rays.
Procedure:
Select sections to represent the average wall thickness, Cut circular sections from two or more areas of the container, and!
vim theth as necessary to give segments ofa size convenient for‘mounting in the Spectrophotorneter, After cutting, woah an
iy each specimen, taking care to avoid scratching the surfaces. I the specimien is too small to cover the opening inthe spec
mee holder, mask the uncovered portion of the opening with opaque paper or masking tape, provided that the length of the
‘specimen i greater than that ofthe sitin the spectrophotometer, Immediately tefore counting in the specimen heldér, wipe
the specimen with lens tissue, Mount the specimen with the aid of a tacky wax, or by other convenient means, taking coe to
‘yoic leasing fingerprints or other marks on the surfaces through wich light must pas Pace the section‘ the specropha-
{ehroter with Its cyingrical axis parallel to the plane ofthe sit and approximately centered with respect tothe sl. Wher prop-
erly placed, the light beam is normal to the surface of the section, and reflection losses are at a minimum,
Continuously measure the transmittance of the section with reference to‘airin the spectral region of interest with a records
ing instrument or at intevais of about 20 nm witha manual instrument, in the region of 290.430 nin.
Limits
‘The observed spectral transmission dois hot excecd the limits given in Tobie. for containers intended for parenteral use.
‘The observed spectral tansmission for plastic containers for products intended for oral or topical administration does not ex:
ceed 10% at any wavelength in the range 290-450 rant.
{etter det ding spas and potas cere sy temas tothe flong puso oLASTM temainal, 103 Bar Hor Dive it
Spnsionecen Pa 19428-2959: “Staneers Tet ethos of Testor Hake ane Lume Hanamtanceof-anspaten Press, AST Metiod GYaDS Trek
“Sandee cues for Computing the Cor of Shiels By Ling the Cle Sten, ASTM Matnad C108.0
8 (67F) Containers—Performance Testing / Physical Tests UsP 40
‘Table 3. Limits for Plastic Casses to Vi
‘Maximum Percentage of Spectral
"Transmission at Any
Nominal size "Wavelengths
nt) between 290 and 45D aim
1 30
2 4s
3 40
aw. 35
2. 2
0 a5
INoTe~Any. container ofa size intermodiate to those sted above exhibits a spectral transmission not greater'than that of the
‘ext large-size container listed in Table 3, For containers larger than $0 mi, the limits for 50 mL apply]
GLOSSARY
ter: Formed, fiddled, and sealed plastic orfeil dome that contains the capsute or tablet (usually asinighe-unit or unite
dose),
Low-barrier blister: Blisters made from low-tvarier materials formed andi sealed so that the moistute vapor transmission
fate wh tested at 40°/75% relative numicity (RH) is greater than 1.0 mg/cavily-lay.
High:banver blister: Blisters made fron» high-barrier material, formed and Sealed so that the moisture Yapor transmission,
«ate when tested at 40°/75%6 RH is less than 1,0 mgicavity-day.
Ultra-high barrier blister: Blisters made from ukira high-barrier material, formed and sealed so that the moisture vapor
‘wansmission cate when tested at 40°/75% RH is fess than 0.08 mg/eaviy-day.
Blister card: A contiguous group of blisters formed and sealed with lid in place. The number of blisters per card common
'y ranges from one to-ten but may be more. The blister card may sometimes be referred to asa packaging s)stem,
Cavity: Formed, idded, and sealed plastic or foil dome (see Biter).
Moisture vapor transmission rate: The steady state moisture vapor transmission in unit time through a packaging sys-
tem, under specific conditions of temperature. and humidity. These test methods use gravimetric measurement to determine
the rate of weight goin as a result of water vapor transmission into the packaging system and subsequent uptake by a des
‘cant enclosed within the packaging system.
Test specimen (or specimen): For multiple-unit containers the bottle fs the test specimen; and for single-unit or unit
dose containers, the-blster card-containing mulkiple blister cavities isthe test sfecimten. For blisters, more than one card {or
spetimen) may be grouped inio a test unit fer conducting the test
‘Test unit: For multipleunit containers, the bottle is the test unit as well as being the test specimen’ and for single-unit or
unit-dose containers, the test unit is a group of test specimens (blister cards) processed together for temperature and humidity
exposure and for weighing at each time point. The purpose ofthe test unit for single-init or unit-dose containers Isto gain the
advantage of additive weight gain resulting from more blister cayities than are present. on a single card. The test uni, when
applied to botties, s used to, mairitain congruence of naming among the three test methods.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- CTP2024 V 2Document55 pagesCTP2024 V 2terminatrixNo ratings yet
- PFD MainDocument6 pagesPFD MainterminatrixNo ratings yet
- Operation CostDocument1 pageOperation CostterminatrixNo ratings yet
- Irritec Flyer Tandem enDocument2 pagesIrritec Flyer Tandem enterminatrixNo ratings yet
- Irritec Flyer Junior enDocument2 pagesIrritec Flyer Junior enterminatrixNo ratings yet
- PFD RevisionDocument4 pagesPFD RevisionterminatrixNo ratings yet
- 8D AnalysisDocument1 page8D AnalysisterminatrixNo ratings yet
- Warranty ANalysisDocument5 pagesWarranty ANalysisterminatrixNo ratings yet
- GORE Magnet Wires DataSheet OG - A4Document4 pagesGORE Magnet Wires DataSheet OG - A4terminatrixNo ratings yet
- FPIP Statement 5Document1 pageFPIP Statement 5terminatrixNo ratings yet
- Friday Amsterdam Bijlmer Arena: Bus Connection N800Document6 pagesFriday Amsterdam Bijlmer Arena: Bus Connection N800terminatrixNo ratings yet
- 101X Catalog 03 Magnet Basics SafetyDocument1 page101X Catalog 03 Magnet Basics SafetyterminatrixNo ratings yet
- 92 Fuel Gas Station - Sta. InesDocument10 pages92 Fuel Gas Station - Sta. InesterminatrixNo ratings yet
- 2019 Digital Packages QuotationsDocument18 pages2019 Digital Packages QuotationsterminatrixNo ratings yet
- Dolores Regala PangilinanDocument3 pagesDolores Regala PangilinanterminatrixNo ratings yet
- Current Archive Folder in : Soulworker Client Version 1.9.13.6 Current Archive Folder in : Soulworker Client Version 1.9.12.4Document1 pageCurrent Archive Folder in : Soulworker Client Version 1.9.13.6 Current Archive Folder in : Soulworker Client Version 1.9.12.4terminatrixNo ratings yet
- Fuel Station SampleDocument2 pagesFuel Station SampleterminatrixNo ratings yet
- Card of Ragnarok Mobile - CloDocument3 pagesCard of Ragnarok Mobile - CloterminatrixNo ratings yet
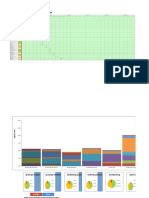
- Yamazumi Chart (Current)Document8 pagesYamazumi Chart (Current)terminatrixNo ratings yet
- Role: Process Engineer: Responsibility 1: Efficiency ImprovementDocument1 pageRole: Process Engineer: Responsibility 1: Efficiency ImprovementterminatrixNo ratings yet
- 03-10 Tom Donnelly DOE For INFORMS NYCDocument37 pages03-10 Tom Donnelly DOE For INFORMS NYCterminatrixNo ratings yet