Professional Documents
Culture Documents
Reaction mechanism-SN1 AND SN2-1 - 231123 - 105832
Reaction mechanism-SN1 AND SN2-1 - 231123 - 105832
Uploaded by
Gaurav Kumar PratapOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reaction mechanism-SN1 AND SN2-1 - 231123 - 105832
Reaction mechanism-SN1 AND SN2-1 - 231123 - 105832
Uploaded by
Gaurav Kumar PratapCopyright:
Available Formats
SCHOLAR’S FORUM
EMPOWERING FUTURE SCHOLARS
IITJEE/NEET/KVPY/NTSE
DPP- REACTION MECHANISM:SN1 AND SN2
1. Which one of the following has maximum nucleophilicity?
CH 3
|
(a) C H 3 (b) NH2 (c) CH3O (d) CH C O
3
|
CH 3
2. Which one of the following has maximum nucleophilicity ?
(a) CH3S (b) C6H5 – O (c) Et3N (d) F
3. Correct arrangement of the following nucleophiles in the order of their nucleophilic strength is–
(a) C6H5O < CH3O < CH3COO < OH (b) CH3COO < C6H5O < CH3O < OH
(c) C6H5O < CH3COO < CH3O < OH (d) CH3COO < C6H5O < OH < CH3O
4. Decreasing order of relative nucleophilicity of the following nucleophiles in protic solvent is –
(a) SH Ac O Ph O O H H 2O (b) SH O H Ph O Ac O H 2 O
(c) SH Ph O O H H 2 O Ac O (d) O H SH Ph O Ac O H 2O
5. CH2-x CH2 CN
NaCN
In the given reaction rate is fastest, when (X) is :
O O
|| ||
(a) – OH (b) – NH2 (c) S OCH 3 (d) O S CH 3
|| ||
O O
6. Which of the following is not expected to be intermediate of the following reaction ?
OH
I
H2O
OH2
(a) (b) (c) (d)
7. What will be the major product of the following reaction?
H CH 3
| | CH3OH,30 C
CH 3 C C CH CH 3
| | |
H CH 3Br
H CH 3 CH 3 H CH 3 OCH3
| | | | | |
(a) CH C C C CH (b) CH C C C CH 3
3 3 3
| | | | | |
OCH 3 H H H CH 3 H
ALIGANJ/ AASHIYANA/ Hazratganj, Lucknow- 7231912196
SCHOLAR’S FORUM
EMPOWERING FUTURE SCHOLARS
IITJEE/NEET/KVPY/NTSE
H CH3 CH3 H CH 3 H
| | | | | |
(c) CH3 C C C CH3 (d) CH3 C C C CH 2
| | | | | | |
H OCH3H H CH 3 H OCH 3
8. Which one of the following compounds will give (d) and (l) form in SN1 reaction (as major product)
CH 3 H C2H5 H
| | | |
(a) CH3 C Br (b) CH C Br (c) CH C Br (d) CH CH C Br
3 3 3
| | | | |
CH 3 C2 H 5 C2H5 CH 3 CH 3
9. Which of the following will be most reactive for SN1 reaction ?
OH
OH
OH
CH OH |
(a) (b) | (c) CH C CH (d)
3 3
C6 H 5 CH C 6 H 5 |
CH 3
10. Which describes the best stereochemical aspects of the following reaction ?
H3 C
H Br
Et Product
Ph
OH
(a) Inversion of configuration occurs at the carbon undergoing substitution.
(b) Retention of configuration occurs at the carbon undergoing substitution.
(c) Racemization occurs at the carbon undergoing substitution.
(d) The carbon undergoing substitution is not stereogenic.
11. CH3
D
HI
product
OH
Identify the major product
CH3
I
I D D D
I
(a) D (b) (c) I (d)
12. Br
H 2 O/Acetone
(A); Product (A) is :
Br
Br OH OH
OH
(a) OH (b) OH (c) (d) Br
13. When the concentration of alkyl halide is tripled and the concentration of O H ion is reduced to
half, the rate of SN2 reaction increase by :
(a) 3 times (b) 2 times (c) 1.5 times (d) 6 times
ALIGANJ/ AASHIYANA/ Hazratganj, Lucknow- 7231912196
SCHOLAR’S FORUM
EMPOWERING FUTURE SCHOLARS
IITJEE/NEET/KVPY/NTSE
14. The decreasing order of rate of SN2 reaction is :
CH 3 C CH 2 Cl CH 3 CH CH 2 Cl
CH3 – Cl || | CH3 CH2 Cl
O CH 3
(i) (ii) (iii) (iv)
(a) IV > III > II > I (b) II > III > I > IV (c) II > I > IV > III (d) none
CH3(CH2)2 – CH2OH X,NaBr,H SO
15. 2 4
Identify X and the mechanism of the reaction
(a) CH3 – CH2 – CH2 – CH2 – Br & SN1 (b) CH3 – CH2 – CH2 – CH2 – Br & SN2
CH 3 CH CH 2 CH 3 &SN 1 CH 3 CH CH 2 CH 3 &SN 2
(c) | (d) |
Br Br
16. In the given reaction : CH3 – CH2 – CH2 – O – CH2 – CH3
HCl
[X] + [Y]
[X] and [Y] will respectively be :
(a) CH3 – CH2 – CH2 OH & CH3 – CH2 – Cl (b) CH3 – CH2 – CH2 – Cl & CH3 – CH2 – OH
(c) CH3 – CH2 – CH2 – Cl & CH2 = CH2 (d) CH3 – CH = CH2 & CH2 = CH2
17. Which one is the strongents nucleiphilic site in the following species ?
O O
4
CH3
1O
3
O S
O O
O2
(a) 1 (b) 2 (c) 3 (d) 4
18. SN1 reaction undergoes through a carbocation intermediate as follows :-
R – X (aq.) R+ (aq.) + X- (aq.) ROH (aq.) + H+ (aq.)
H2O
fast
[R = t – Bu, iso – Pr, Et, Me] (X = Cl, Br, I)
The correct statements are
(i) The decreasing order of rate of SN1 reaction is t – BuX > iso – PrX > EtX > MeX
(ii) The decreasing order of ionization energy is MeX > EtX > iso – PrX > t – BuX
(iii) The decreasing order of energy of activation is t – BuX > iso – PrX > EtX > MeX
(a) I & II are correct (b) I & III are correct (c) II and III are correct (d) I, II & III are correct
ANSWER KEY
1(a) 2(a) 3(d) 4(b) 5(d) 6(a)
7(c) 8(b) 9(d) 10(c) 11(a) 12(a)
13(c) 14(c) 15(b) 16(a) 17(d) 18(a)
ALIGANJ/ AASHIYANA/ Hazratganj, Lucknow- 7231912196
You might also like
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- Organic Chemistry Help! Practice Exam Window For Xula-O1e2Document7 pagesOrganic Chemistry Help! Practice Exam Window For Xula-O1e2Kristia Stephanie BejeranoNo ratings yet
- Organic Chemistry: Nucleophilic Substitution at Saturated CarbonDocument107 pagesOrganic Chemistry: Nucleophilic Substitution at Saturated CarbonMafalda FernandesNo ratings yet
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thDocument8 pagesAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thRaju SinghNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit 07-12-23Deena chemistNo ratings yet
- Chem 2016Document4 pagesChem 2016Shubhankar ChakrabortyNo ratings yet
- SN1 RXNSDocument4 pagesSN1 RXNSAshutosh KolseNo ratings yet
- Exercise - III Subjective Level-I: CH-CHDocument3 pagesExercise - III Subjective Level-I: CH-CHPriyanshu RajNo ratings yet
- PAPER1Document6 pagesPAPER1baidanshulNo ratings yet
- Reaction Mechanism - Revision Live SessionDocument2 pagesReaction Mechanism - Revision Live Sessiontemp93630No ratings yet
- Quiz-Alkyl Halide-MksDocument6 pagesQuiz-Alkyl Halide-MksSatyam AgrawalNo ratings yet
- Selina Concise Chemistry Solutions Class 10 Chapter 12 Organic ChemistryDocument54 pagesSelina Concise Chemistry Solutions Class 10 Chapter 12 Organic ChemistryKrishnaPriyaNo ratings yet
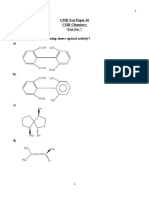
- CSIR Test Paper - 16Document32 pagesCSIR Test Paper - 16Vineeth V TNo ratings yet
- DPP-8 Text Solution 20230717095820Document4 pagesDPP-8 Text Solution 20230717095820ansarinaved8920No ratings yet
- JEE Main Organic Compound Containing Halogens Important QuestionsDocument15 pagesJEE Main Organic Compound Containing Halogens Important QuestionsRuchitha VNo ratings yet
- Quiz Alkyl Halide Mks 01Document7 pagesQuiz Alkyl Halide Mks 01navya.updNo ratings yet
- Super Sixer 6 IsomerismDocument4 pagesSuper Sixer 6 IsomerismKartik YadavNo ratings yet
- C1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesDocument84 pagesC1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesBlack InsomniaNo ratings yet
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- GoodgDocument19 pagesGoodgTusharNo ratings yet
- GRiGNARD REAGENT!!Document22 pagesGRiGNARD REAGENT!!GazalNo ratings yet
- DPP 3 Electronic EffectDocument3 pagesDPP 3 Electronic EffectUsha SherkhaneNo ratings yet
- Tutorial 1Document5 pagesTutorial 1nurqistina hanani HananNo ratings yet
- Class Test-6 - Carboxylic Acid - Amines JEE Adv - CC - AnsDocument6 pagesClass Test-6 - Carboxylic Acid - Amines JEE Adv - CC - Ansbruh pogNo ratings yet
- Neet Sample Paper: Max. Marks: 180 Duration: 3 HrsDocument38 pagesNeet Sample Paper: Max. Marks: 180 Duration: 3 HrsShiv soniNo ratings yet
- Haloalkane and Haloarenes DPP 6Document3 pagesHaloalkane and Haloarenes DPP 6Dharmvir TantyNo ratings yet
- Ape Assignment 3Document7 pagesApe Assignment 3Atharva KulkarniNo ratings yet
- Me Me CL BR CH-CH CH Oh: O PCLDocument3 pagesMe Me CL BR CH-CH CH Oh: O PCLAkhil JamwalNo ratings yet
- Goc QueDocument10 pagesGoc QueMahesh JagtapNo ratings yet
- DPP-12 Text Solution 20230717095958Document4 pagesDPP-12 Text Solution 20230717095958ansarinaved8920No ratings yet
- Acidicity Basicity & H - Bonding TautomerismDocument10 pagesAcidicity Basicity & H - Bonding TautomerismRaju SinghNo ratings yet
- OC Carbonyl Compound EDocument25 pagesOC Carbonyl Compound Eamankumarsingh4141sNo ratings yet
- Chapter 6Document17 pagesChapter 6ayush.rai1068No ratings yet
- Exercise StereochemistryDocument4 pagesExercise StereochemistryPuvaneswary LoganathanNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- Alde & Ket-4Document14 pagesAlde & Ket-4Anusha SinghalNo ratings yet
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 13thDocument16 pagesAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 13thRaju SinghNo ratings yet
- Alcohols IIT PACEDocument19 pagesAlcohols IIT PACEDushyant GuptaNo ratings yet
- Or-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WADocument23 pagesOr-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WAsuryasaiNo ratings yet
- CY2102Document3 pagesCY2102Prarabdha SharmaNo ratings yet
- Research PaperDocument16 pagesResearch PaperShounak DuttaNo ratings yet
- Acidity Basicity TautomerismDocument16 pagesAcidity Basicity TautomerismDpNo ratings yet
- Dhisha Jee Mains and Neet-185-206Document22 pagesDhisha Jee Mains and Neet-185-206jeevavelayyaNo ratings yet
- Organic Chem Mcqs PDFDocument18 pagesOrganic Chem Mcqs PDFDIPA NAGNo ratings yet
- Unit 8R - Oxygen Containing Organic Compounds Practice Problems PDFDocument7 pagesUnit 8R - Oxygen Containing Organic Compounds Practice Problems PDFFirdausia Rahma PutriNo ratings yet
- I Am Sharing 'Assignment-3 Organic' With YouDocument28 pagesI Am Sharing 'Assignment-3 Organic' With YouKriti GargNo ratings yet
- CHM3201 Tutorial 1 Basic ConceptsDocument3 pagesCHM3201 Tutorial 1 Basic ConceptsAkmalZharifAbdullahNo ratings yet
- Assignment For 1 Puc Students ChemistryDocument3 pagesAssignment For 1 Puc Students Chemistrykrishnabrunda72No ratings yet
- Nomeculture 2Document3 pagesNomeculture 2Ram KNo ratings yet
- Tutorial 1: Catenation'Document4 pagesTutorial 1: Catenation'AdellNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticeSeungwoo ParkNo ratings yet
- Naming Organic Compounds Practice: ExercisesDocument3 pagesNaming Organic Compounds Practice: ExercisesSk MukulNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticepangilinankrisallenNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticejhingajuriNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticeSiddanth SatishNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticeLiu YiNo ratings yet
- Aldehydes Ketones: Subjective ProblemsDocument16 pagesAldehydes Ketones: Subjective ProblemsBhaskar AnandNo ratings yet
- Chem0871 NamingOrganicCompoundsPracticeDocument3 pagesChem0871 NamingOrganicCompoundsPracticemudzungatshivhase2006No ratings yet
- Nucleophilic Substitution Reaction - Nur Aisyah ImranDocument3 pagesNucleophilic Substitution Reaction - Nur Aisyah ImranNur ImranNo ratings yet
- Chapter 5a - Alkyl Halides (Substitution Reaction) PDFDocument13 pagesChapter 5a - Alkyl Halides (Substitution Reaction) PDFsachinNo ratings yet
- Null 1Document12 pagesNull 1Bijoy BiswasNo ratings yet
- .In Ypage: Name Reactions (Organic Chemistry)Document12 pages.In Ypage: Name Reactions (Organic Chemistry)Sai SidharthNo ratings yet
- Haloalkane - by @MadXAbhiOfficial - HandBookDocument8 pagesHaloalkane - by @MadXAbhiOfficial - HandBooksr.enterprises.4651No ratings yet
- Organic Chemistry IIDocument7 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- Electrophilic SubstitutionDocument4 pagesElectrophilic SubstitutionPrasad BidweNo ratings yet
- Name Reactions Chemistry All PDFDocument12 pagesName Reactions Chemistry All PDFSundram KumarNo ratings yet
- Reactions of Halogenoalkanes: Www. .CO - UKDocument15 pagesReactions of Halogenoalkanes: Www. .CO - UKcharlesma123No ratings yet
- Homework Assignments Chapter-6: Alkyl Halides-Substitution and Elimination ReactionsDocument14 pagesHomework Assignments Chapter-6: Alkyl Halides-Substitution and Elimination ReactionsandrewNo ratings yet
- 1 BenzeneDocument14 pages1 BenzeneAleep YoungBaeNo ratings yet
- Name Reactions - Chemistrypage PDFDocument12 pagesName Reactions - Chemistrypage PDFManojNo ratings yet
- (IIT JEE IITJEE Chemistry) Nitin D Gaikwad - Organic Reaction Mechanism Through Problem Solving Approach Nitin D Gaikwad K.T.H.M. College Nashik ISBN 978-93-5267-423-7-K.T.H.M. College Nashik (2019)Document204 pages(IIT JEE IITJEE Chemistry) Nitin D Gaikwad - Organic Reaction Mechanism Through Problem Solving Approach Nitin D Gaikwad K.T.H.M. College Nashik ISBN 978-93-5267-423-7-K.T.H.M. College Nashik (2019)Nia100% (1)
- Substitution Elimination FlowchartDocument2 pagesSubstitution Elimination FlowchartAyah Al-AnaniNo ratings yet
- Reaction MechanismDocument20 pagesReaction MechanismPalash ChawhanNo ratings yet
- Name Reactions - Chemistrypage PDFDocument12 pagesName Reactions - Chemistrypage PDFPuneet DeshwaniNo ratings yet
- Module 2.3 NGPDocument3 pagesModule 2.3 NGPIshaan ChaturvediNo ratings yet
- Misconceptions On SN1 SN2 ReactionsDocument3 pagesMisconceptions On SN1 SN2 ReactionsEdcademiaNo ratings yet
- HDA Short NotesDocument4 pagesHDA Short Notesadithaj.2006220No ratings yet
- Name Reactions - Chemistrypage PDFDocument12 pagesName Reactions - Chemistrypage PDFchinmayaNo ratings yet
- .In Ypage: Name Reactions (Organic Chemistry)Document12 pages.In Ypage: Name Reactions (Organic Chemistry)vedangNo ratings yet
- Xicbse Che Asst 2 AnsDocument3 pagesXicbse Che Asst 2 Anstanishkakannan3253No ratings yet
- SN1 SN2 PDFDocument16 pagesSN1 SN2 PDFPrajval ChauhanNo ratings yet
- Nucleophilic Substitution (S 1/S 2) Elimination (E1/E2)Document5 pagesNucleophilic Substitution (S 1/S 2) Elimination (E1/E2)Marck LyonNo ratings yet
- CH CH H C OK: Classes of S 1, S 2, E1 and E2 ReactionsDocument2 pagesCH CH H C OK: Classes of S 1, S 2, E1 and E2 ReactionsBereket ShimelisNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Sustitucion, Eliminacion OrganicaDocument16 pagesSustitucion, Eliminacion OrganicajeremiasNo ratings yet
- Module 2.3 NGP PDFDocument3 pagesModule 2.3 NGP PDFIshaan ChaturvediNo ratings yet
- Chemistry 2Document4 pagesChemistry 2loretta00No ratings yet