Professional Documents
Culture Documents
A Fisica Parte 2
A Fisica Parte 2
Uploaded by
Evandro Aguiar Nascimento0 ratings0% found this document useful (0 votes)
4 views1 pageA Fisica da Termografia Pate 2
Copyright
© © All Rights Reserved
Available Formats
TXT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA Fisica da Termografia Pate 2
Copyright:
© All Rights Reserved
Available Formats
Download as TXT, PDF, TXT or read online from Scribd
Download as txt, pdf, or txt
0 ratings0% found this document useful (0 votes)
4 views1 pageA Fisica Parte 2
A Fisica Parte 2
Uploaded by
Evandro Aguiar NascimentoA Fisica da Termografia Pate 2
Copyright:
© All Rights Reserved
Available Formats
Download as TXT, PDF, TXT or read online from Scribd
Download as txt, pdf, or txt
You are on page 1of 1
Humans cannot directly see infrared radiation.
They can, how-
ever, feel or sense it in the form of thermal radiation, which pro-
duces warming of the surface of the skin. The skin of the human
cheek below the eye can normally sense the increased thermal
radiation of a warm palm held at a distance of 2 inches (5 cm) at a
room temperature of 70�F (21�C).5
In physics, the word �heat� can be either a noun or a verb: a warm
body will heat (verb) a cooler body, increasing the heat (noun) of the
cooler body. As a noun, heat refers to the total amount of random
kinetic energy the molecules of a substance have at any given moment,
measured in units of joules (J). Heat as a verb refers to energy transfer
from a warm to a cold body. The cooler body increases its internal
energy, while the warmer body decreases its internal energy until
the temperatures of the two bodies become equal. This heat transfer
does not stop when the two bodies are at the same temperature. The
bodies continue to radiate heat toward one another, but the radiation
balances out, so the net energy transfer is zero.
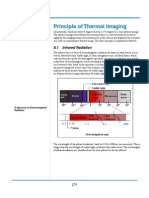
Objects at high temperatures are capable of emitting radia-
tion with shorter wavelengths than infrared, including visible
and ultraviolet light. Steel at 1500�C (2732�F, 1773�K) has a peak
emitted wavelength of 1.64 �m in the near-infrared band, yet it
appears white hot to the human eye due to emitting some radia-
tion in the visible range (see the radiation curves in Figure 2.5).
For bodies having physiologic surface temperatures, however, the
radiated energy peaks within the 7�14 �m octave of the electro-
magnetic spectrum and even the shorter wavelengths emitted are
invisible to the eye. The human body emits its peak radiation in
the 9�10 �m part of the spectrum, thus imagers with detectors
sensitive to this radiation are preferred in medical thermogra-
phy. Imagers capable of viewing other infrared wavelengths are of
more practical use in industrial and scientific research settings.
The term �emissivity� in thermal imaging refers to the ability
of a surface to emit or absorb radiation within 9�10-�m infrared
range. The range of emissivity runs from 1.0 (the ideal emitter/
absorber) to 0.0 (the ideal reflector). In physics, a blackbody is an
object that has an emissivity of 1.0. None of the incident radiation
is reflected from or passes through a perfect blackbody. Thus, a
blackbody completely absorbs all radiant energy reaching it, and
simultaneously emits radiant energy. If not otherwise heated or
cooled, it eventually attains a temperature equal to its environ-
ment and then maintains that energy balance.
The physical opposite of an infrared blackbody is a surface that
is extremely shiny and reflective to infrared. Mathematically,
emissivity (e) plus reflectance (R) add up to unity: (e + R = 1).
Polished aluminum or brass surfaces have an infrared emissivity
of 3% (e = 0.03), meaning that they reflect 97% of incoming rays. A
silver-coated glass mirror has a similar high infrared reflectance.
Liquid water, though it may appear to have visible reflectivity, has
an infrared emissivity of 0.95�0.96 (perpendicular to the water
surface), almost as emissive as human skin.6
There are four fundamental mechanisms by which the heat
generated by the human body is transferred to its surroundings,
as discussed in the following text.
You might also like
- A Level OCR Chemistry A - Paper 1 June 2023Document27 pagesA Level OCR Chemistry A - Paper 1 June 2023kapedispurs33% (3)
- Heat 4e Chap12 LectureDocument43 pagesHeat 4e Chap12 LectureSubho SamantaNo ratings yet
- Ophthalmic Dispensing Revision Guide: First Year Part OneFrom EverandOphthalmic Dispensing Revision Guide: First Year Part OneRating: 4 out of 5 stars4/5 (3)
- Lab ReportDocument16 pagesLab ReportSandra Enn Bahinting100% (1)
- HUMAN THERMOGRAPHY 2Document1 pageHUMAN THERMOGRAPHY 2Evandro Aguiar NascimentoNo ratings yet
- Unit 5 - Heat and Mass TransferDocument19 pagesUnit 5 - Heat and Mass TransferSALMAN FARIS CPNo ratings yet
- Programme: Doctor of Physical Therapy: CourseDocument16 pagesProgramme: Doctor of Physical Therapy: CourseAppzStarNo ratings yet
- TRE Radiation FormsDocument11 pagesTRE Radiation FormsDamisha DamishaNo ratings yet
- A Fisica Parte 3Document1 pageA Fisica Parte 3Evandro Aguiar NascimentoNo ratings yet
- L-7 Overview of Blackbody RadiationDocument6 pagesL-7 Overview of Blackbody Radiationsg6034209No ratings yet
- Blackbody Radiation, But Sometimes Also Hyphenated, As in Black-Body RadiationDocument14 pagesBlackbody Radiation, But Sometimes Also Hyphenated, As in Black-Body Radiationrokib410No ratings yet
- Black BodyDocument8 pagesBlack BodyMatihas DerejeNo ratings yet
- Heat Transfer GME221 Eight LectureDocument36 pagesHeat Transfer GME221 Eight Lecturej.a smartNo ratings yet
- Black-Body Radiation - Wikipedia, The Free EncyclopediaDocument7 pagesBlack-Body Radiation - Wikipedia, The Free Encyclopediadonodoni0008No ratings yet
- Introduction To Thermal Imaging: Joseph E. Osowski, PEDocument18 pagesIntroduction To Thermal Imaging: Joseph E. Osowski, PEashokmmmNo ratings yet
- Illumination Notes NM PDFDocument17 pagesIllumination Notes NM PDFhimNo ratings yet
- Lesson 6 - Radiation - Part IIDocument19 pagesLesson 6 - Radiation - Part IIKier John Michael AtreroNo ratings yet
- Infrared Thermography On Ocular SurfaceDocument21 pagesInfrared Thermography On Ocular SurfaceEdson silvaNo ratings yet
- Black Body RadiationDocument5 pagesBlack Body Radiationmahapatraabinas34No ratings yet
- Handout 05 Thermal RadiationDocument6 pagesHandout 05 Thermal RadiationBaja TechinNo ratings yet
- Chapter 11. Fundamental of RadiationDocument29 pagesChapter 11. Fundamental of RadiationDayanidiNo ratings yet
- Lecture34 1Document38 pagesLecture34 1vamsikrishna14No ratings yet
- IR1 L1 Thermal WorldDocument17 pagesIR1 L1 Thermal WorldMohan RajNo ratings yet
- Principle of Thermal ImagingDocument2 pagesPrinciple of Thermal ImagingDodik NugrohoNo ratings yet
- Chapter 12 Fundamentals of Thermal RadiationDocument45 pagesChapter 12 Fundamentals of Thermal RadiationKAYAIRA TATENo ratings yet
- The Concept of RadiationDocument4 pagesThe Concept of RadiationNayly IzzahNo ratings yet
- Fundamentals of Thermal RadiationDocument42 pagesFundamentals of Thermal RadiationEMMANUEL DELOS SANTOSNo ratings yet
- Thermal RadiationDocument11 pagesThermal Radiationgozombie43No ratings yet
- Lecture Remote Sensing 008 ThermalDocument29 pagesLecture Remote Sensing 008 Thermalcurious aimNo ratings yet
- Radiation Heat TransferDocument14 pagesRadiation Heat Transferniyasking0123No ratings yet
- Electrotherapy 2Document138 pagesElectrotherapy 2nagyrashad123No ratings yet
- Heat Transfer by Radiation: EAT RansferDocument27 pagesHeat Transfer by Radiation: EAT RansferRajiv DubeyNo ratings yet
- Thermography Course TranscriptDocument7 pagesThermography Course TranscriptjoshuaNo ratings yet
- 07 RadiationDocument13 pages07 RadiationMhelveneNo ratings yet
- Heat Transfer by RadiationDocument2 pagesHeat Transfer by Radiationyesuplus20% (1)
- Thermal Resistance PDFDocument4 pagesThermal Resistance PDFImtiaz AlamNo ratings yet
- CSE(AIML)_14230823053_SOUMI_HAZRADocument11 pagesCSE(AIML)_14230823053_SOUMI_HAZRAsoumihazra30No ratings yet
- Midle Test A. Black Body RadiationDocument2 pagesMidle Test A. Black Body RadiationErna EternaNo ratings yet
- Alex Ekwueme Federal University Ndufu Alike Ikwo: RadiationDocument10 pagesAlex Ekwueme Federal University Ndufu Alike Ikwo: RadiationMiracle Chibuike OdiNo ratings yet
- Modes of Heat TransferDocument6 pagesModes of Heat TransferfaisalNo ratings yet
- PHYSICS REPORT Blackbody RadiationDocument10 pagesPHYSICS REPORT Blackbody RadiationScribdTranslationsNo ratings yet
- Lecture Thermography PresentationDocument30 pagesLecture Thermography PresentationDhruv SahniNo ratings yet
- Fundamentals of Thermal RadiationDocument23 pagesFundamentals of Thermal Radiationverify nameNo ratings yet
- Infrared Pyrometer HandbookDocument19 pagesInfrared Pyrometer Handbookjoyous leeNo ratings yet
- The Emergence of InfraredDocument1 pageThe Emergence of InfraredEvandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 4Document2 pagesHISTORIA DA TERMOGRAFIA 4Evandro Aguiar NascimentoNo ratings yet
- Phy101 - Note 2Document9 pagesPhy101 - Note 2Kikelomo AjibadeNo ratings yet
- 20bit0054 Phytheory Da 2Document5 pages20bit0054 Phytheory Da 2arpitNo ratings yet
- BASIC PRINCIPLES Non Contact Temperature MeasurementDocument40 pagesBASIC PRINCIPLES Non Contact Temperature MeasurementAkul BrigacNo ratings yet
- 3.laws of Radiation and Their Relevance in RemoteDocument20 pages3.laws of Radiation and Their Relevance in RemoteSujata SarkarNo ratings yet
- Solar Radiation Electromagnetic Radiation Spectrum.: Student Sheet 1Document5 pagesSolar Radiation Electromagnetic Radiation Spectrum.: Student Sheet 1Iqra KhanNo ratings yet
- Report On Human ComfortDocument8 pagesReport On Human ComfortBrijesh KumarNo ratings yet
- Thermography PDFDocument12 pagesThermography PDFAnthonio MJNo ratings yet
- RESUME 1-4 - Satria Mandala Putra - I - J011221096Document12 pagesRESUME 1-4 - Satria Mandala Putra - I - J011221096Satria MandalaNo ratings yet
- Optris Basic Brochure PDFDocument40 pagesOptris Basic Brochure PDFUmair Gul KhanNo ratings yet
- Infrared Thermography 1Document10 pagesInfrared Thermography 1Prince Yasp100% (1)
- Technology in Science: InvestigateDocument11 pagesTechnology in Science: InvestigatePaola GarciaNo ratings yet
- Black Body: Navigation SearchDocument32 pagesBlack Body: Navigation SearchAishwarya RaviNo ratings yet
- Theoretical BackgroundDocument2 pagesTheoretical BackgroundPatrice Pauline TamoriaNo ratings yet
- (2.3) C - Transfer of Thermal Energy - RadiationDocument2 pages(2.3) C - Transfer of Thermal Energy - Radiationzahra1No ratings yet
- For Red Hot Blot to Blue Singing Whale: A look into thermodynamics and the evolution of speciesFrom EverandFor Red Hot Blot to Blue Singing Whale: A look into thermodynamics and the evolution of speciesNo ratings yet
- Learn How to Protect & Restore Yourself from Negative EnergyFrom EverandLearn How to Protect & Restore Yourself from Negative EnergyNo ratings yet
- HUMAN THERMOGRAPHY 13Document2 pagesHUMAN THERMOGRAPHY 13Evandro Aguiar NascimentoNo ratings yet
- Human Thermography 15Document1 pageHuman Thermography 15Evandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 1Document1 pageHISTORIA DA TERMOGRAFIA 1Evandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 3Document1 pageHISTORIA DA TERMOGRAFIA 3Evandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 4Document2 pagesHISTORIA DA TERMOGRAFIA 4Evandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 2Document1 pageHISTORIA DA TERMOGRAFIA 2Evandro Aguiar NascimentoNo ratings yet
- Thermal Imager Calibration 1Document1 pageThermal Imager Calibration 1Evandro Aguiar NascimentoNo ratings yet
- HISTORIA DA TERMOGRAFIA 5Document2 pagesHISTORIA DA TERMOGRAFIA 5Evandro Aguiar NascimentoNo ratings yet
- Thermal Imager Calibration 3Document1 pageThermal Imager Calibration 3Evandro Aguiar NascimentoNo ratings yet
- Fundamentos Parte 5Document2 pagesFundamentos Parte 5Evandro Aguiar NascimentoNo ratings yet
- A Fisica Parte 8Document1 pageA Fisica Parte 8Evandro Aguiar NascimentoNo ratings yet
- A History of Thermology and ThermographyDocument2 pagesA History of Thermology and ThermographyEvandro Aguiar NascimentoNo ratings yet
- A Fisica Parte 9Document1 pageA Fisica Parte 9Evandro Aguiar NascimentoNo ratings yet
- Fundamentos Parte 1Document2 pagesFundamentos Parte 1Evandro Aguiar NascimentoNo ratings yet
- A Fisica Parte 7Document1 pageA Fisica Parte 7Evandro Aguiar NascimentoNo ratings yet
- Lecture 04 - Interference and ApplicationsDocument27 pagesLecture 04 - Interference and ApplicationsArc ZeroNo ratings yet
- Edexcel International AS Physics: Interference & Stationary WavesDocument30 pagesEdexcel International AS Physics: Interference & Stationary WavesAhmed MahmoudNo ratings yet
- L2-Heat Conduction Equation in Rectangular-Cylinderical & Spherical CoordinatesDocument19 pagesL2-Heat Conduction Equation in Rectangular-Cylinderical & Spherical CoordinatesskNo ratings yet
- Lecture 11 12Document5 pagesLecture 11 12Muhammad Attiq Ur RehmanNo ratings yet
- Gas Law HomeworkDocument6 pagesGas Law Homeworkneo cultureNo ratings yet
- DISTILLATIONDocument6 pagesDISTILLATIONAvinash KulkarniNo ratings yet
- Oscillarty MotionDocument20 pagesOscillarty MotionAhmed NegmNo ratings yet
- Sci4 ST2 Q3Document2 pagesSci4 ST2 Q3KayelNo ratings yet
- Resonance of A Closed Air ColumnDocument11 pagesResonance of A Closed Air ColumnHo Ping100% (6)
- Sir Zaheer'S Academy: Third OrderDocument19 pagesSir Zaheer'S Academy: Third OrderJawad NaqviNo ratings yet
- Week3 AssignmentDocument2 pagesWeek3 AssignmentJainil PatelNo ratings yet
- 3RD Quarter Exam Grade 7 ScienceDocument3 pages3RD Quarter Exam Grade 7 ScienceHugot Reactions100% (1)
- Intregration PT 2Document4 pagesIntregration PT 2Augustin LouisNo ratings yet
- WavesDocument6 pagesWavesShrinay ChandraNo ratings yet
- Home Work 17 SolutionsDocument6 pagesHome Work 17 Solutions洪健勛No ratings yet
- What Is Sound?Document63 pagesWhat Is Sound?Ma. Teresa BajaoNo ratings yet
- Revision Notes For Checkpoint ChemistryDocument13 pagesRevision Notes For Checkpoint ChemistryKostas TampakisNo ratings yet
- Heat Exchanger DesignDocument83 pagesHeat Exchanger DesignPhượng NguyễnNo ratings yet
- Thermodynamics NotesDocument16 pagesThermodynamics Notesmuneerahmed7130100% (1)
- Heat & Mass Transfer Jan 2014Document2 pagesHeat & Mass Transfer Jan 2014Prasad C M100% (1)
- Experimental Verification of Einstein'S Photoelectric EquationDocument11 pagesExperimental Verification of Einstein'S Photoelectric EquationNayana MDNo ratings yet
- Me 701 Heat and Mass Transfer Dec 2020Document2 pagesMe 701 Heat and Mass Transfer Dec 2020Shyam Sunder PrasadNo ratings yet
- Mbeya University of Science and TechnologyDocument13 pagesMbeya University of Science and TechnologyEmmanuel ChachaNo ratings yet
- Al 22 Kinematics GraphsDocument12 pagesAl 22 Kinematics GraphsSenuja ChammithaNo ratings yet
- Experiment - Reflection, Transmission and Refraction of MicrowavesDocument8 pagesExperiment - Reflection, Transmission and Refraction of Microwavesmuzahir.ali.baloch2021No ratings yet
- Thermal Transmission (Winter)Document20 pagesThermal Transmission (Winter)Robin Charles SamuelNo ratings yet
- Energy Dissipators and Hydraulic Jump by Willi H. Hager (Auth.)Document298 pagesEnergy Dissipators and Hydraulic Jump by Willi H. Hager (Auth.)Cecilia GalvezNo ratings yet
- Chapter12 Rotational DynamicsDocument9 pagesChapter12 Rotational Dynamicstariq8physicsNo ratings yet