Professional Documents
Culture Documents
Inibation
Inibation
Uploaded by
RajatCopyright:
Available Formats
You might also like
- Enzyme InhibitionDocument8 pagesEnzyme InhibitionfayeNo ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme Inhibitionprincesssilver419No ratings yet
- What Are Enzyme Inhibitors?: EnzymesDocument5 pagesWhat Are Enzyme Inhibitors?: EnzymesRica NorcioNo ratings yet
- Enzyme Inhibition and ToxicityDocument12 pagesEnzyme Inhibition and ToxicityDaniel OmolewaNo ratings yet
- Botany J-Adhikary Enzymology 4Document9 pagesBotany J-Adhikary Enzymology 4Dharmesh R.DNo ratings yet
- Enzyme InhibitionDocument25 pagesEnzyme Inhibitionmehahe2105No ratings yet
- Lecture 10 - BCH-701 Enzymes Part 3Document12 pagesLecture 10 - BCH-701 Enzymes Part 3Rana FurqanNo ratings yet
- Inhibition: Maria Roceline P. Sandoy 2MT01Document15 pagesInhibition: Maria Roceline P. Sandoy 2MT01Roceline SandoyNo ratings yet
- Enzyme InhibitorDocument1 pageEnzyme InhibitorANo ratings yet
- Enzyme InhibitorDocument15 pagesEnzyme InhibitorNatalia BalbinottNo ratings yet
- Enzyme Inhibitors As Poisons: Presented By: Aman Pratap Singh Roll No: 20 63rd BatchDocument6 pagesEnzyme Inhibitors As Poisons: Presented By: Aman Pratap Singh Roll No: 20 63rd Batchcosmosman306No ratings yet
- Enzyme Inhibitor: Enzyme Activators Bind To Enzymes and Increase Their Enzymatic ActivityDocument2 pagesEnzyme Inhibitor: Enzyme Activators Bind To Enzymes and Increase Their Enzymatic ActivityAhmed AbuelfutuhNo ratings yet
- Biochemistry 1 Lesson 22 (Enzyme Inhibitors)Document43 pagesBiochemistry 1 Lesson 22 (Enzyme Inhibitors)ansuben961No ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme InhibitiongeffkryptonNo ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme InhibitiongeffkryptonNo ratings yet
- Irreversible InhibitorsDocument5 pagesIrreversible InhibitorsbelabelawNo ratings yet
- Activators and Inhibitors (Enzyme)Document17 pagesActivators and Inhibitors (Enzyme)ronojoysengupta100% (2)
- Chapter Iv EnzymesDocument6 pagesChapter Iv EnzymesJohn TacordaNo ratings yet
- Lecture Note - Enzyme InhibitorDocument10 pagesLecture Note - Enzyme InhibitorBright ChimezieNo ratings yet
- Chapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'tDocument21 pagesChapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'telsaNo ratings yet
- L-3 Enzyme InhibitorsDocument4 pagesL-3 Enzyme InhibitorsLiyakath AliNo ratings yet
- 8.1 (2.5 HL)Document21 pages8.1 (2.5 HL)SudeNo ratings yet
- 8.1 MetabolismDocument24 pages8.1 MetabolismbashayerstupidNo ratings yet
- Enzyme Inhibitors. Definition, Classification, and Main PropertiesDocument3 pagesEnzyme Inhibitors. Definition, Classification, and Main PropertiesAkmaral TleubaevaNo ratings yet
- Enzymes Terms and MeaningDocument3 pagesEnzymes Terms and MeaningSubash GunasekaranNo ratings yet
- 44 Enzyme InhibitionDocument13 pages44 Enzyme InhibitionMafe HernandezNo ratings yet
- Enzyme InhibitionDocument49 pagesEnzyme InhibitionJaden StanislausNo ratings yet
- 15-16, Enzyme Inhibition and DeactivationDocument59 pages15-16, Enzyme Inhibition and DeactivationS. AnsaryNo ratings yet
- Biochemestry 3 AssignmentDocument3 pagesBiochemestry 3 Assignmentkeithotieno5No ratings yet
- Metabolism (HL)Document26 pagesMetabolism (HL)Sapreen KaurNo ratings yet
- Enzyme InhibitionDocument11 pagesEnzyme Inhibitionshekinah656No ratings yet
- c1.1 EnzymesDocument36 pagesc1.1 Enzymeshwqhd asjdhuaNo ratings yet
- Tugas 2 BiokatalisisDocument7 pagesTugas 2 BiokatalisisKirstie ImeldaNo ratings yet
- Enzyme RegulationDocument3 pagesEnzyme RegulationrubiomaryfloryvielNo ratings yet
- Enzyme InhibitorsDocument27 pagesEnzyme InhibitorsNAUSHEEN SULTANANo ratings yet
- All of Those Enzymes Up and Metabolize As Fast As Possible! As It TurnsDocument11 pagesAll of Those Enzymes Up and Metabolize As Fast As Possible! As It TurnsshahzebNo ratings yet
- Topic 8.1: Metabolism: Metabolic PathwaysDocument1 pageTopic 8.1: Metabolism: Metabolic PathwaysKaleab GebreegizabiherNo ratings yet
- EnzymeDocument5 pagesEnzymeSepta DewiNo ratings yet
- Enzyme Inhibition/Enzyme InhibitorsDocument5 pagesEnzyme Inhibition/Enzyme InhibitorsFaria bukhariNo ratings yet
- Chapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Document4 pagesChapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20yplsinghNo ratings yet
- Chapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Document4 pagesChapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20lokeshmujalde197No ratings yet
- Introduction To EnzymesDocument10 pagesIntroduction To EnzymesShreeraj BadgujarNo ratings yet
- Types of Enzyme Inhibitors Modified Ocober 2022Document45 pagesTypes of Enzyme Inhibitors Modified Ocober 2022DENNIS murageNo ratings yet
- ENZYMESDocument24 pagesENZYMESAmjad AlmousawiNo ratings yet
- Enzyme Inhibition: Mechanisms and ScopeDocument35 pagesEnzyme Inhibition: Mechanisms and ScopeJason SteelNo ratings yet
- Avcn T2Document17 pagesAvcn T2Huân Lê Văn LâmNo ratings yet
- Topic 8 MetabolismDocument23 pagesTopic 8 MetabolismSammy MicahNo ratings yet
- Principles of Biochemistry - Enzymes - Wikibooks, Open Books For An Open WorldDocument19 pagesPrinciples of Biochemistry - Enzymes - Wikibooks, Open Books For An Open WorldstanleybrowncoleNo ratings yet
- Types of Enzyme InhibitionDocument3 pagesTypes of Enzyme InhibitionDavid LevisteNo ratings yet
- Regulation of Enzyme ActivityDocument26 pagesRegulation of Enzyme ActivityWeird WorldNo ratings yet
- EnzymesDocument2 pagesEnzymesHannah BercasioNo ratings yet
- 04 2020enzyme InhibitionDocument20 pages04 2020enzyme InhibitionDharmesh R.DNo ratings yet
- Lecture-18 Chapter 3Document18 pagesLecture-18 Chapter 3ktrone88No ratings yet
- OrganicMedicinals Session3 SASDocument15 pagesOrganicMedicinals Session3 SASChristaly Nejar NabuabNo ratings yet
- Enzyme RegulationDocument3 pagesEnzyme RegulationgeffkryptonNo ratings yet
- Enzyme InhibitionDocument17 pagesEnzyme InhibitionazwelljohnsonNo ratings yet
- Competitive InhibitorsDocument2 pagesCompetitive InhibitorsParis JarrettNo ratings yet
- Enzymes, Vitamins Presentation Part II ScriptDocument5 pagesEnzymes, Vitamins Presentation Part II ScriptViragNo ratings yet
- Amyloid Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandAmyloid Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Amoxicillin: A Beginner's 20-Minute Quick Guide Overview on its Use Cases to Treat Bacterial Infection and Side EffectsFrom EverandAmoxicillin: A Beginner's 20-Minute Quick Guide Overview on its Use Cases to Treat Bacterial Infection and Side EffectsNo ratings yet
Inibation
Inibation
Uploaded by
RajatOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Inibation
Inibation
Uploaded by
RajatCopyright:
Available Formats
Powered by AI
Bing
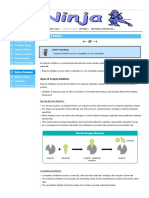
Enzyme inhibition is the process of reducing or stopping the activity of an enzyme by the presence of certain
molecules called inhibitors. There are two main types of enzyme inhibition: reversible and irreversible.
Reversible inhibition occurs when the inhibitor binds to the enzyme through weak, non-covalent
interactions, such as hydrogen bonds, ionic bonds, or hydrophobic interactions. This means that the
inhibitor can be removed from the enzyme, allowing it to regain its activity. Reversible inhibitors can be
classified into four subtypes: competitive, non-competitive, uncompetitive, and mixed inhibitors,
depending on how and where they bind to the enzyme or the enzyme-substrate complex.
Irreversible inhibition occurs when the inhibitor binds to the enzyme through strong, covalent bonds,
such as disulfide bonds, alkylation, or acylation. This means that the inhibitor permanently modifies the
enzyme, rendering it inactive unless the bond is broken. Irreversible inhibitors can be classified into two
subtypes: suicide inhibitors and allosteric inhibitors, depending on whether they bind to the active site or
a different site of the enzyme.
Enzyme inhibition is an important biological phenomenon that regulates various metabolic pathways, protects
cells from harmful enzymes, and serves as a potential therapeutic strategy. For more information, you can
check out these web pages:
Difference Between Reversible and Irreversible Enzyme Inhibition
Enzyme Inhibition - Definition, Types, Mechanism, Examples
Enzyme Inhibition - Chemistry LibreTexts
You might also like
- Enzyme InhibitionDocument8 pagesEnzyme InhibitionfayeNo ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme Inhibitionprincesssilver419No ratings yet
- What Are Enzyme Inhibitors?: EnzymesDocument5 pagesWhat Are Enzyme Inhibitors?: EnzymesRica NorcioNo ratings yet
- Enzyme Inhibition and ToxicityDocument12 pagesEnzyme Inhibition and ToxicityDaniel OmolewaNo ratings yet
- Botany J-Adhikary Enzymology 4Document9 pagesBotany J-Adhikary Enzymology 4Dharmesh R.DNo ratings yet
- Enzyme InhibitionDocument25 pagesEnzyme Inhibitionmehahe2105No ratings yet
- Lecture 10 - BCH-701 Enzymes Part 3Document12 pagesLecture 10 - BCH-701 Enzymes Part 3Rana FurqanNo ratings yet
- Inhibition: Maria Roceline P. Sandoy 2MT01Document15 pagesInhibition: Maria Roceline P. Sandoy 2MT01Roceline SandoyNo ratings yet
- Enzyme InhibitorDocument1 pageEnzyme InhibitorANo ratings yet
- Enzyme InhibitorDocument15 pagesEnzyme InhibitorNatalia BalbinottNo ratings yet
- Enzyme Inhibitors As Poisons: Presented By: Aman Pratap Singh Roll No: 20 63rd BatchDocument6 pagesEnzyme Inhibitors As Poisons: Presented By: Aman Pratap Singh Roll No: 20 63rd Batchcosmosman306No ratings yet
- Enzyme Inhibitor: Enzyme Activators Bind To Enzymes and Increase Their Enzymatic ActivityDocument2 pagesEnzyme Inhibitor: Enzyme Activators Bind To Enzymes and Increase Their Enzymatic ActivityAhmed AbuelfutuhNo ratings yet
- Biochemistry 1 Lesson 22 (Enzyme Inhibitors)Document43 pagesBiochemistry 1 Lesson 22 (Enzyme Inhibitors)ansuben961No ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme InhibitiongeffkryptonNo ratings yet
- Enzyme InhibitionDocument3 pagesEnzyme InhibitiongeffkryptonNo ratings yet
- Irreversible InhibitorsDocument5 pagesIrreversible InhibitorsbelabelawNo ratings yet
- Activators and Inhibitors (Enzyme)Document17 pagesActivators and Inhibitors (Enzyme)ronojoysengupta100% (2)
- Chapter Iv EnzymesDocument6 pagesChapter Iv EnzymesJohn TacordaNo ratings yet
- Lecture Note - Enzyme InhibitorDocument10 pagesLecture Note - Enzyme InhibitorBright ChimezieNo ratings yet
- Chapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'tDocument21 pagesChapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'telsaNo ratings yet
- L-3 Enzyme InhibitorsDocument4 pagesL-3 Enzyme InhibitorsLiyakath AliNo ratings yet
- 8.1 (2.5 HL)Document21 pages8.1 (2.5 HL)SudeNo ratings yet
- 8.1 MetabolismDocument24 pages8.1 MetabolismbashayerstupidNo ratings yet
- Enzyme Inhibitors. Definition, Classification, and Main PropertiesDocument3 pagesEnzyme Inhibitors. Definition, Classification, and Main PropertiesAkmaral TleubaevaNo ratings yet
- Enzymes Terms and MeaningDocument3 pagesEnzymes Terms and MeaningSubash GunasekaranNo ratings yet
- 44 Enzyme InhibitionDocument13 pages44 Enzyme InhibitionMafe HernandezNo ratings yet
- Enzyme InhibitionDocument49 pagesEnzyme InhibitionJaden StanislausNo ratings yet
- 15-16, Enzyme Inhibition and DeactivationDocument59 pages15-16, Enzyme Inhibition and DeactivationS. AnsaryNo ratings yet
- Biochemestry 3 AssignmentDocument3 pagesBiochemestry 3 Assignmentkeithotieno5No ratings yet
- Metabolism (HL)Document26 pagesMetabolism (HL)Sapreen KaurNo ratings yet
- Enzyme InhibitionDocument11 pagesEnzyme Inhibitionshekinah656No ratings yet
- c1.1 EnzymesDocument36 pagesc1.1 Enzymeshwqhd asjdhuaNo ratings yet
- Tugas 2 BiokatalisisDocument7 pagesTugas 2 BiokatalisisKirstie ImeldaNo ratings yet
- Enzyme RegulationDocument3 pagesEnzyme RegulationrubiomaryfloryvielNo ratings yet
- Enzyme InhibitorsDocument27 pagesEnzyme InhibitorsNAUSHEEN SULTANANo ratings yet
- All of Those Enzymes Up and Metabolize As Fast As Possible! As It TurnsDocument11 pagesAll of Those Enzymes Up and Metabolize As Fast As Possible! As It TurnsshahzebNo ratings yet
- Topic 8.1: Metabolism: Metabolic PathwaysDocument1 pageTopic 8.1: Metabolism: Metabolic PathwaysKaleab GebreegizabiherNo ratings yet
- EnzymeDocument5 pagesEnzymeSepta DewiNo ratings yet
- Enzyme Inhibition/Enzyme InhibitorsDocument5 pagesEnzyme Inhibition/Enzyme InhibitorsFaria bukhariNo ratings yet
- Chapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Document4 pagesChapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20yplsinghNo ratings yet
- Chapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Document4 pagesChapter 6 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20lokeshmujalde197No ratings yet
- Introduction To EnzymesDocument10 pagesIntroduction To EnzymesShreeraj BadgujarNo ratings yet
- Types of Enzyme Inhibitors Modified Ocober 2022Document45 pagesTypes of Enzyme Inhibitors Modified Ocober 2022DENNIS murageNo ratings yet
- ENZYMESDocument24 pagesENZYMESAmjad AlmousawiNo ratings yet
- Enzyme Inhibition: Mechanisms and ScopeDocument35 pagesEnzyme Inhibition: Mechanisms and ScopeJason SteelNo ratings yet
- Avcn T2Document17 pagesAvcn T2Huân Lê Văn LâmNo ratings yet
- Topic 8 MetabolismDocument23 pagesTopic 8 MetabolismSammy MicahNo ratings yet
- Principles of Biochemistry - Enzymes - Wikibooks, Open Books For An Open WorldDocument19 pagesPrinciples of Biochemistry - Enzymes - Wikibooks, Open Books For An Open WorldstanleybrowncoleNo ratings yet
- Types of Enzyme InhibitionDocument3 pagesTypes of Enzyme InhibitionDavid LevisteNo ratings yet
- Regulation of Enzyme ActivityDocument26 pagesRegulation of Enzyme ActivityWeird WorldNo ratings yet
- EnzymesDocument2 pagesEnzymesHannah BercasioNo ratings yet
- 04 2020enzyme InhibitionDocument20 pages04 2020enzyme InhibitionDharmesh R.DNo ratings yet
- Lecture-18 Chapter 3Document18 pagesLecture-18 Chapter 3ktrone88No ratings yet
- OrganicMedicinals Session3 SASDocument15 pagesOrganicMedicinals Session3 SASChristaly Nejar NabuabNo ratings yet
- Enzyme RegulationDocument3 pagesEnzyme RegulationgeffkryptonNo ratings yet
- Enzyme InhibitionDocument17 pagesEnzyme InhibitionazwelljohnsonNo ratings yet
- Competitive InhibitorsDocument2 pagesCompetitive InhibitorsParis JarrettNo ratings yet
- Enzymes, Vitamins Presentation Part II ScriptDocument5 pagesEnzymes, Vitamins Presentation Part II ScriptViragNo ratings yet
- Amyloid Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandAmyloid Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Amoxicillin: A Beginner's 20-Minute Quick Guide Overview on its Use Cases to Treat Bacterial Infection and Side EffectsFrom EverandAmoxicillin: A Beginner's 20-Minute Quick Guide Overview on its Use Cases to Treat Bacterial Infection and Side EffectsNo ratings yet