Professional Documents
Culture Documents
Types of Chemical Attacks On Concrete Structures - The Constructor
Types of Chemical Attacks On Concrete Structures - The Constructor
Uploaded by
sagar khanalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Types of Chemical Attacks On Concrete Structures - The Constructor
Types of Chemical Attacks On Concrete Structures - The Constructor
Uploaded by
sagar khanalCopyright:
Available Formats
VIP ⌂ Home VIP $ Earn Money Type Search Words 🔍 Sign In
$ Earn Money
Shop Merchandise
Types of Chemical Attacks on Concrete Structures
📖 Questions
⌂ Home / Concrete Technology / Concrete Cracks
Ask Question
✎ Write Article
Building
Concrete
Construction
How To Guide
Geotechnical
Structural ✎
×
Engineering Get Noti cations
Surveying
Tips
Contents: [show]
Introduction
When a concrete structure is prone to chemical actions its durability gets
a ected. The chemicals may cause cracking of concrete, volume change
and deterioration of structure. The life of structure reduces and it can lead
to failure of structures. Di erent types of chemical attacks and their e ects
on concrete structures are explained below.
Scaffolding Manufacturer
Q235 Q345 Steel
Click Here To Explore More. Large In
Stocks, Complete Speci cations, Quality
Assurance.
hunanworld.com
OPEN
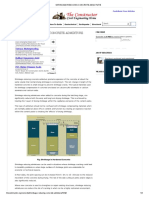
Types of chemical attacks on concrete structures
Following are the di erent chemical actions on concrete structures
& Ad Free! EXPLORE VIP Membership
Sulphate attack
Chloride attack
Alkali aggregate reaction
Carbonation
Acid attack
Sulphate attack on concrete
Most of the soil types contains sulphates in the form of calcium,
magnesium, sodium, ammonium and potassium. They occur in soil or
ground water. When a concrete structure is built on these types of soils,
they may attack the concrete.
Ad
First-class Batching Plant
Batching Plant With Twin-shaft&SICOMA Concrete
Mixer, 5%Discount This Month.
Xinfeng Machinery Get Quote
Generally sulphates in solid form do not attack the concrete severely but
when they are in liquid form they pass into the voids of concrete and react
with hydrated cement products. Calcium sulphate causes minimum
damage because of its low solubility while magnesium sulphate causes
maximum damage.
Most of the sulphates attacks calcium hydroxide and hydrated calcium
aluminates present in the concrete and results in changing the volume of
cement paste in concrete. Hence deterioration of concrete structure takes
place. Along with calcium hydroxide, Magnesium sulphate also reacts with
hydrated calcium silicate and makes concrete into powdered mass.
Precautions
Concrete with low water cement ratio is less a ected by magnesium
sulphate while high water cement ratio concrete is highly a ected.
Sulphate-resisting Portland cement should be used where sulphates are
present in the soil, water or atmosphere and come into contact with the
concrete.
Super-sulphated cement, made from blast furnace slag, can also be used
although it is not widely available. This cement can resist the highest 📖 Popular Articles
concentrations of sulphates.
Concrete Mix Design
Ak Calculation for M20, M25, M30
Chloride attack on concrete Concrete with
Chloride attack on concrete is one of the important aspects of durability of Calculate Quantities of
concrete. It primarily a ects the reinforcement of concrete and cause Materials for Concrete -Cement,
Sand, Aggregates
corrosion. Chlorides can be introduced into the concrete either during or
after construction as follows.
Quantity of Cement and Sand
Calculation in Mortar
1. Before construction Chlorides can be admitted in admixtures containing
calcium chloride, through using mixing water contaminated with salt water Preparation of Bar Bending
or improperly washed marine aggregates. Schedule (BBS) and Its
Advantages
2. After construction Chlorides in salt or sea water, in airborne sea spray and
from de-icing salts can attack permeable concrete causing corrosion of Civil Engineering Tips - Points
reinforcement. to Remember for Civil Site
The chloride in the presence of water and oxygen reacts with alkaline
protected layer around the reinforcement and removes it.
📖 Recent Articles
8 Ways COVID-19 is Changing
Residential Designs
How to Construct a LEED-
Certi ed House?
How to Identify Reinforcing
Bars?
What are the Types of Cladding
Installation Systems?
Alkali-Aggregate reaction on concrete
What are the Materials Used for
Alkali aggregate reaction is the chemical reaction between alkali in cement
Ferrocement Construction?
and silica content of aggregates. Hence it can also be called as Alkali Silica
reaction. When this reaction takes place, a gel like substance is formed
which absorbs water and volume of concrete will increase. This increasing
volume develops cracking and disintegration of concrete.
BS8110: Part 2, clause 6.2.5.4, states that the Alkali Silica reaction only
occurs when the following are present together:
1. Concrete with high moisture level.
2. When cement contains high alkali content in it.
3. Aggregate with Alkali reactive constituents.
Popular Answers
What Software are used to
Estimate Project Time and
Quantity in Construction?
What is the di erence between
Tender and Contract
Documents?
How to calculate the quantity of
water for a given concrete mix.?
What are Bearing Capacity
Values of Di erent Types of
Soil?
Form work stripping time Vs
Compressive strength of
Concrete
Precautions
The code recommends that the following precautions be taken if
uncertainty exists:
1. Reduce the Saturation of concrete.
2. Usage of Low alkali Portland cement.
3. Use replacement cementitious materials such as blast furnace slag or
pulverized fuel ash. Most normal aggregates behave satisfactorily.
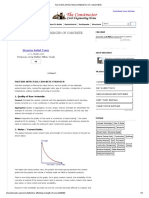
Carbonation in concrete
When the carbon dioxide from the atmosphere penetrates into concrete
and reacted with calcium hydroxide to form calcium carbonate then this
process is called carbonation.
In general concrete with high alkali content form a protective layer around
the reinforcement. But when the carbon dioxide changes into dilute
carbonic acid it reduces the alkalinity as a result the corrosion of
reinforcement takes place.
Carbonated concrete has a pH value of 8.3 while the passivation of steel
starts at a pH value of 9.5. The depth of Carbonation in good dense concrete
is about 3 mm at an early stage and may increase to 6–10 mm after 30–40
years. Poor concrete may have a depth of Carbonation of 50 mm after say
6–8 years.
The rate of Carbonation depends on
Time
Depth of cover
Concrete density
Cement content
Water-to-cement ratio
The presence of cracks
The depth of carbonation is determined using the solution of
phenolphthalein in diluted alcohol. When the solution is applied the Non –
carbonation zones becomes pink in color and remaining uncolored portion
is termed as carbon a ected zone.
Acid attack on concrete
Acids can attack concrete easily since concrete is not fully resistant against
acids. Some acids like oxalic acid, phosphoric acids are not harmful to the
concrete. Calcareous aggregates are more a ected by acids while siliceous
aggregates are good resistant.
The damage level is purely depends upon the pH of the acid solution.
Damage is very severe if the pH value is very low. If they reach
reinforcement through crack or pores, they will cause corrosion of bars and
cracking of concrete will occur.
Prevention of acid attack
To prevent acid attack good dense concrete with adequate cover is required
and sulphate-resistant cements should be used.
DOWNLOAD: Strengthening of Chemically Deteriorated Building
Share This Article Facebook
Sadanandam Anupoju ✓
EDITOR
Sadananda is a Civil Engineer and is an Author, Editor and Partner of The Constructor since
2016.
← Previous article Next article →
Site and Landscape Planning for Green SMART STRUCTURES AND MATERIALS
Building Construction
Related Posts
What are the Materials Used for What are the Products of Cement
Ferrocement Construction? Hydration? [PDF]
2 Comments
Arief Wibowo
Added a comment on July 13, 2014 at 4:33 pm
The knowledge of:
TYPES OF CHEMICAL ATTACKS ON CONCRETE STRUCTURES
🔒 Login to Reply Share
Patricia Dulaney
Added a comment on February 13, 2013 at 6:05 am
We might thought that concretes are very sturdy that no one can break
it unless it's done purposely. Now, after reading this I now know that
those noted above, should be observed so that our concrete ooring and
post will not be destroyed. -http://www.oregononsite.com/
🔒 Login to Reply Share
Leave a comment
You must login or register to add a new comment.
Login with:
Secured by OneAll Social Login
📁 Know More 📁 Connect With Us
Search ...
⌂ Home Ask Question
Articles
About Us ✎ Write Article
Contact Us VIP Subscriptions
Search
Privacy Policy Popular Questions
Our Team
© 2009-2020 The Constructor. All Rights Reserved.
You might also like
- Taller ContaminantesDocument11 pagesTaller ContaminantesDaniel F Amado0% (1)
- Life 2e BrE Inter SB U01AB Day-1Document29 pagesLife 2e BrE Inter SB U01AB Day-1Quỳnh NhưNo ratings yet
- Accelerating Water-Reducing Concrete AdmixturesDocument2 pagesAccelerating Water-Reducing Concrete AdmixturesDS20CE017Bhaskar WabhitkarNo ratings yet
- Functions of Concrete AdmixturesDocument2 pagesFunctions of Concrete AdmixturesDS20CE017Bhaskar WabhitkarNo ratings yet
- Standard Codes For Concrete AdmixturesDocument2 pagesStandard Codes For Concrete AdmixturesDS20CE017Bhaskar WabhitkarNo ratings yet
- Carbonation of Concrete StructuresDocument2 pagesCarbonation of Concrete StructuresDS20CE017Bhaskar WabhitkarNo ratings yet
- How Does Contact With Aluminum Affect Concrete - Concrete Construction MagazineDocument1 pageHow Does Contact With Aluminum Affect Concrete - Concrete Construction MagazineHerdisNo ratings yet
- Chloride Attack On Concrete StructuresDocument2 pagesChloride Attack On Concrete StructuresDS20CE017Bhaskar WabhitkarNo ratings yet
- Corrosion Inhibiting AdmixturesDocument2 pagesCorrosion Inhibiting AdmixturesDS20CE017Bhaskar WabhitkarNo ratings yet
- Topics in Concrete: MenuDocument2 pagesTopics in Concrete: MenuShaneNo ratings yet
- Concrete Design Task 01Document19 pagesConcrete Design Task 01DahamNo ratings yet
- Durability PDFDocument18 pagesDurability PDFnafiyadNo ratings yet
- NotesDocument31 pagesNotesErika Nell LachicaNo ratings yet
- MyNotes ConcreteDocument18 pagesMyNotes ConcreteKarl Si AkoNo ratings yet
- Shrinkage Reducing Concrete AdmixtureDocument3 pagesShrinkage Reducing Concrete AdmixtureDS20CE017Bhaskar WabhitkarNo ratings yet
- Shrinkage Reducing Concrete AdmixtureDocument3 pagesShrinkage Reducing Concrete AdmixtureDS20CE017Bhaskar WabhitkarNo ratings yet
- Granolithic Flooring - The Construction CivilDocument2 pagesGranolithic Flooring - The Construction CivilKailas AnandeNo ratings yet
- Division of Concrete - GROUP 1Document42 pagesDivision of Concrete - GROUP 1Vincepaulo CadanoNo ratings yet
- Achieving Durability For Concrete StructuresDocument2 pagesAchieving Durability For Concrete StructuresDS20CE017Bhaskar WabhitkarNo ratings yet
- 5 TaDocument79 pages5 TaHussein El BeqaiNo ratings yet
- Week 6 ConcreteDocument26 pagesWeek 6 Concreteisrarisroo10No ratings yet
- Retarding Water-Reducing Concrete AdmixtureDocument2 pagesRetarding Water-Reducing Concrete AdmixtureDS20CE017Bhaskar WabhitkarNo ratings yet
- Retarding Water-Reducing Concrete AdmixtureDocument2 pagesRetarding Water-Reducing Concrete AdmixtureDS20CE017Bhaskar WabhitkarNo ratings yet
- Concrete Repair-SikaDocument72 pagesConcrete Repair-SikaMurthy BabuNo ratings yet
- SCC-somesh Siddharth-A1988520002Document20 pagesSCC-somesh Siddharth-A1988520002Somesh SiddharthNo ratings yet
- Concrete Technology NotesDocument36 pagesConcrete Technology Notesshalu R FNo ratings yet
- Effect of Admixture On Properties of ConDocument12 pagesEffect of Admixture On Properties of ConTahmidtuhinNo ratings yet
- Concrete Repair and ProtectionDocument33 pagesConcrete Repair and ProtectionakdassNo ratings yet
- Alkali Aggregate Reaction in Concrete - Types, Causes and EffectsDocument2 pagesAlkali Aggregate Reaction in Concrete - Types, Causes and EffectssudhakarrrrrrNo ratings yet
- Chapter 1 - IntroductionDocument17 pagesChapter 1 - IntroductionVincent Nava100% (1)
- 1.1 Concrete: Partial Replacement of Coarse Aggregate With Ceramic Tile 2019-2020Document25 pages1.1 Concrete: Partial Replacement of Coarse Aggregate With Ceramic Tile 2019-2020Naveen S100% (2)
- 04 Reinforced Concrete Constituents 01Document14 pages04 Reinforced Concrete Constituents 01S. M. ZAHIDUR RAHMAN 1301129No ratings yet
- Types of Concrete - WikipediaDocument11 pagesTypes of Concrete - WikipediaAndi SilalahiNo ratings yet
- 04 Reinforced Concrete Constituents 01Document14 pages04 Reinforced Concrete Constituents 01AhsanurRahmanShuvoNo ratings yet
- MaterialDocument80 pagesMaterialNaol AdugnaNo ratings yet
- Reinforced Concrete Design :introductionDocument52 pagesReinforced Concrete Design :introductionRandy PolicarpioNo ratings yet
- On ConcreteDocument102 pagesOn Concreteblg watersupplyNo ratings yet
- Log InJoinDocument19 pagesLog InJoinrajukg1231544No ratings yet
- Types of Sealants Used For Joints in Buildings - Properties, Uses, WorkingDocument6 pagesTypes of Sealants Used For Joints in Buildings - Properties, Uses, WorkingJustin MusopoleNo ratings yet
- Chapter 1 - Concrete Material & MixtureDocument10 pagesChapter 1 - Concrete Material & MixtureNezrinNo ratings yet
- Factors Affecting Strength of ConcreteDocument4 pagesFactors Affecting Strength of ConcreteDS20CE017Bhaskar WabhitkarNo ratings yet
- Concrete Properties Archives - The ConstructorDocument10 pagesConcrete Properties Archives - The ConstructorSeungcheol ChoiNo ratings yet
- Water ProofingDocument7 pagesWater ProofingMoureen ViveroNo ratings yet
- Synapsis Bablu KumarDocument25 pagesSynapsis Bablu Kumarbd.steptslNo ratings yet
- HJKKKDocument27 pagesHJKKKTafaraNo ratings yet
- Structural Design of Interlocking Concrete Paving Block: E. Palanikumar Pothuganti Uday KumarDocument4 pagesStructural Design of Interlocking Concrete Paving Block: E. Palanikumar Pothuganti Uday Kumarivanhendriprasetyo 127No ratings yet
- TOS Theory AnswersDocument6 pagesTOS Theory AnswersRizzel DiasNo ratings yet
- Construction and Building Materials: Lei Jiang, Ditao NiuDocument11 pagesConstruction and Building Materials: Lei Jiang, Ditao NiuXtem AlbNo ratings yet
- Consmat For MidtermDocument16 pagesConsmat For MidtermArnel Leewen VillalonNo ratings yet
- Cement and Cement ConcreteDocument22 pagesCement and Cement ConcreteGayadhar SahuNo ratings yet
- Steel Rust - Article 2 PDFDocument11 pagesSteel Rust - Article 2 PDFBalasubramanian MahadevanNo ratings yet
- A Civil Engg. Final Year Training Report On Residential Building ConstructionDocument9 pagesA Civil Engg. Final Year Training Report On Residential Building ConstructionSharath PVNo ratings yet
- Effect of Cement Composition in Concrete On Resisting External Sulfate AttackDocument11 pagesEffect of Cement Composition in Concrete On Resisting External Sulfate AttackSelvanNo ratings yet
- Factors Affecting Durability of Lightweight Concrete and Its RemediesDocument5 pagesFactors Affecting Durability of Lightweight Concrete and Its RemediesfelixNo ratings yet
- 13 Concrete Repairs 4CDocument17 pages13 Concrete Repairs 4CMina SaflorNo ratings yet
- Principles of RC Design - Lecture 3Document19 pagesPrinciples of RC Design - Lecture 3ezartsandprintNo ratings yet
- CMT Chapter5 ConcreteDocument12 pagesCMT Chapter5 ConcreteElvin AsanasNo ratings yet
- 236763-Article Text-572027-1-10-20221124Document7 pages236763-Article Text-572027-1-10-20221124WalterGentileNo ratings yet
- BUILDING MATERIALS AND ASSEMBLIES (Handout) - BSCE-3BDocument24 pagesBUILDING MATERIALS AND ASSEMBLIES (Handout) - BSCE-3BArianne Mae De Vera GallonNo ratings yet
- Project Note M.tech Phase 1Document18 pagesProject Note M.tech Phase 1Rocks KiranNo ratings yet
- Example of RL Transfer FormatDocument1 pageExample of RL Transfer Formatsagar khanalNo ratings yet
- 41two-Peg-Test FormatDocument1 page41two-Peg-Test Formatsagar khanalNo ratings yet
- Major Traverse Distance Data FormatDocument1 pageMajor Traverse Distance Data Formatsagar khanalNo ratings yet
- 39 40HCRMinorTraverseDocument2 pages39 40HCRMinorTraversesagar khanalNo ratings yet
- WSEreport 075bce174Document17 pagesWSEreport 075bce174sagar khanalNo ratings yet
- Chapter 8&9 FinalDocument91 pagesChapter 8&9 Finalsagar khanalNo ratings yet
- Final-Chapter-6.1-6.6 1Document98 pagesFinal-Chapter-6.1-6.6 1sagar khanalNo ratings yet
- Chapter-6 (6 6 2 2-6 9 3)Document107 pagesChapter-6 (6 6 2 2-6 9 3)sagar khanalNo ratings yet
- Chapter 4Document102 pagesChapter 4sagar khanalNo ratings yet
- Ahb Example Amba System: Technical Reference ManualDocument222 pagesAhb Example Amba System: Technical Reference ManualJinsNo ratings yet
- Industry Profile: K.S.S.K LTD, HaveriDocument42 pagesIndustry Profile: K.S.S.K LTD, HaveriManju PalegarNo ratings yet
- CommunityDocument4 pagesCommunityJennyNo ratings yet
- Setting Product Strategy: Marketing Management, 13 EdDocument43 pagesSetting Product Strategy: Marketing Management, 13 EdEucharistia Yacoba NugrahaNo ratings yet
- Buddha and His ContemporariesDocument7 pagesBuddha and His ContemporariesAlok VermaNo ratings yet
- Teaching Guide 2 1 4Document58 pagesTeaching Guide 2 1 4Anu KhanNo ratings yet
- Resonant DC-DC Converting Controller Santhosh112Document21 pagesResonant DC-DC Converting Controller Santhosh112SanthoshNo ratings yet
- McDonalds Supplier Workplace AccountabilityDocument3 pagesMcDonalds Supplier Workplace Accountabilityjamil voraNo ratings yet
- Ibr CalculationsDocument9 pagesIbr CalculationsoperationmanagerNo ratings yet
- DIP DharDocument17 pagesDIP Dharabhi_1mehrotaNo ratings yet
- Jacob BohmeDocument12 pagesJacob BohmeTimothy Edwards100% (1)
- Ansi Asa S3.1 1999 R2008Document27 pagesAnsi Asa S3.1 1999 R2008fco2312100% (1)
- A General Mathematical Model For Two-Parameter GeneratingDocument15 pagesA General Mathematical Model For Two-Parameter GeneratingFranciscoGarciaNo ratings yet
- REPORT OptimizCapacityDocument24 pagesREPORT OptimizCapacityJosé Agustín Moreno DíazNo ratings yet
- For Teachers of English: Vol. 29 No. 1 - January/June 2022 ISSN 0120-5927Document243 pagesFor Teachers of English: Vol. 29 No. 1 - January/June 2022 ISSN 0120-5927Luisa CalderónNo ratings yet
- Rtx3 Wireless Expansion V5.3: Reference and Installation ManualDocument24 pagesRtx3 Wireless Expansion V5.3: Reference and Installation ManualCatalin HoratiuNo ratings yet
- First FruitsDocument32 pagesFirst FruitsRodolfo Babilonia Jr100% (3)
- Statement of IntentDocument4 pagesStatement of IntentteanNo ratings yet
- List of Approved Students of Ehsaas Undergraduate Scholarships Program (2020-2021)Document19 pagesList of Approved Students of Ehsaas Undergraduate Scholarships Program (2020-2021)Khubaib Ur RehmanNo ratings yet
- Src419X 192-Khz Stereo Asynchronous Sample-Rate Converters: 1 Features 3 DescriptionDocument45 pagesSrc419X 192-Khz Stereo Asynchronous Sample-Rate Converters: 1 Features 3 DescriptionNguyễn QuangNo ratings yet
- Copper Tube HandbookDocument66 pagesCopper Tube HandbookRafael Leonardo GomezNo ratings yet
- DR .Sharma Ji-05Document1 pageDR .Sharma Ji-05arunNo ratings yet
- TDAE Product Tank: HSD Storage Tank HSD Storage TankDocument7 pagesTDAE Product Tank: HSD Storage Tank HSD Storage TankAnonymous p0iwBoNo ratings yet
- Ekc 204aDocument24 pagesEkc 204aPreot Andreana CatalinNo ratings yet
- 09 - Chapter 2 PDFDocument207 pages09 - Chapter 2 PDFAarthi PriyaNo ratings yet
- Genkie 2Document39 pagesGenkie 2cherryl carmelotesNo ratings yet
- Marketing Communications in The Digital AgeDocument49 pagesMarketing Communications in The Digital Age2m shoppingNo ratings yet
- PR2 Qtr1 Module 2Document50 pagesPR2 Qtr1 Module 2mememew suppasitNo ratings yet