Professional Documents
Culture Documents
4.11 Exercise 1 - Mass Spectra
4.11 Exercise 1 - Mass Spectra
Uploaded by
muzamilstudy2468Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4.11 Exercise 1 - Mass Spectra
4.11 Exercise 1 - Mass Spectra
Uploaded by
muzamilstudy2468Copyright:
Available Formats
13.
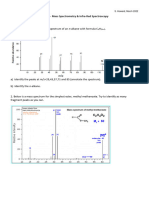
11 EXERCISE 1 – Mass spectra
1. Butanoic acid and methylpropanoic acid give slightly different mass spectra. Both
give a molecular ion peak at m/z = 88, but butanoic acid give six other peaks
whereas methylpropanoic only gives four.
a) State the species responsible for the four other peaks in the mass spectrum
of butanoic acid and write equations to show their formation from the
molecular ion.
b) Suggest which one of these peaks will not be present in a mass spectrum
of methylpropanoic acid, giving a reason for your choice.
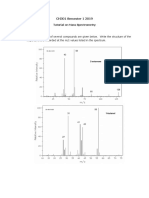
2. Suggest how pentan-2-one and pentan-3-one could be distinguished in a mass
spectrum. Write equations to show the formation of any important fragment ions.
3. Write equations to show the formation of at least two species giving intense peaks
in the mass spectra of each of the following molecules:
a) pentane
b) ethyl ethanoate
c) propanoic acid
d) pentanal
You might also like
- 5.5.1 PracticeDocument12 pages5.5.1 PracticeSid MathurNo ratings yet
- Fragmenttaion-Halides To Learning ResourcesDocument11 pagesFragmenttaion-Halides To Learning ResourcesShinu DiazNo ratings yet
- 1H NMR Problem SetDocument5 pages1H NMR Problem Setfishfeeder1No ratings yet
- Tutorial 3 (Mass Spectrometry & Infra-Red Spectroscopy)Document6 pagesTutorial 3 (Mass Spectrometry & Infra-Red Spectroscopy)Ahmed ZakyNo ratings yet
- Tutorial Mass Spectrometry PDFDocument4 pagesTutorial Mass Spectrometry PDFArjun MaharajNo ratings yet
- Tutorial Mass Spectrometry PDFDocument4 pagesTutorial Mass Spectrometry PDFArjun MaharajNo ratings yet
- AminesDocument12 pagesAminesMadhu SomavarapuNo ratings yet
- Chem273 Take Home LabDocument12 pagesChem273 Take Home LabMentari Permata HatiNo ratings yet
- Chem 112A Homework 1Document4 pagesChem 112A Homework 1Shyam BhaktaNo ratings yet
- Isomers ExercisesDocument3 pagesIsomers ExercisesScribdTranslationsNo ratings yet
- ApDocument1 pageApsaiyamdeep SaiyamdeepNo ratings yet
- Glance Through The Entire Questions and Solve The Easiest Problems FirstDocument4 pagesGlance Through The Entire Questions and Solve The Easiest Problems FirstRutul JainNo ratings yet
- Exam 1 Org - ChemDocument12 pagesExam 1 Org - ChemOlah BosenkoNo ratings yet
- 243B NMR Problem SetDocument5 pages243B NMR Problem SetRushabhDaulatNo ratings yet
- Spec Prob Set 315 CurrentDocument20 pagesSpec Prob Set 315 CurrentGershom MbeweNo ratings yet
- Assignment 4.corrected VersionDocument6 pagesAssignment 4.corrected VersionIgneel CreedNo ratings yet
- National 5 Chemistry Unit 2 Nature's ChemistryDocument18 pagesNational 5 Chemistry Unit 2 Nature's ChemistryDoraNo ratings yet
- Reductions in Organic ChemistryDocument13 pagesReductions in Organic ChemistryNikola PudjaNo ratings yet
- Cy4202 21-22 EndDocument3 pagesCy4202 21-22 EndAakash BanerjeeNo ratings yet
- Clayden2e Problems Ch08Document4 pagesClayden2e Problems Ch08aggelisgeorge8546No ratings yet
- Problems For Chapter 8: Problem 1Document4 pagesProblems For Chapter 8: Problem 1aggelisgeorge8546No ratings yet
- Organic Chem. IV. BCH 2210 Cat 1 and Ii INSTRUCTIONS: 1. Answer ALL Questions in Section A (40 Marks)Document19 pagesOrganic Chem. IV. BCH 2210 Cat 1 and Ii INSTRUCTIONS: 1. Answer ALL Questions in Section A (40 Marks)Brian MbuguaNo ratings yet
- 8.26.0 ORGANIC CHEMISTRY MY Revised NC NOTESDocument52 pages8.26.0 ORGANIC CHEMISTRY MY Revised NC NOTESUpenyu MachingambiNo ratings yet
- Clayden2e Problems Ch08Document4 pagesClayden2e Problems Ch08Eniola Kaikohanakami AjayiNo ratings yet
- 2015 Apr-Biochemistry & Physical ChemistryDocument2 pages2015 Apr-Biochemistry & Physical ChemistryD PrajnaNo ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- He Following Are The List of Aliphatic Compounds, According To The Number of Carbon Atoms They Contain. Complete The Table BelowDocument17 pagesHe Following Are The List of Aliphatic Compounds, According To The Number of Carbon Atoms They Contain. Complete The Table BelowJohn Rafael DiazNo ratings yet
- Chem 121 - Prob Set 1Document1 pageChem 121 - Prob Set 1Jayson FranciscoNo ratings yet
- Optional Area Examination Analytical ChemistryDocument4 pagesOptional Area Examination Analytical ChemistryMohamed DahmaneNo ratings yet
- OC Part B QuestionsDocument10 pagesOC Part B QuestionsSunanda 2004No ratings yet
- 11.3D Analytical TechniquesDocument58 pages11.3D Analytical TechniquesЕлнур ИкимбаевNo ratings yet
- Assignment 1Document3 pagesAssignment 1鄒大安No ratings yet
- 유기2 19 중간Document2 pages유기2 19 중간rkfladlwkdNo ratings yet
- Afinidad Protónica y Sitios de Protonación en P-NitroanilinaDocument8 pagesAfinidad Protónica y Sitios de Protonación en P-NitroanilinaFabian Loor CadenaNo ratings yet
- Chemistry 318: Ir, MS, Uv, NMR SpectrosDocument17 pagesChemistry 318: Ir, MS, Uv, NMR Spectrosaamer_shahbaaz0% (4)
- Review NotesDocument7 pagesReview NotesKatie MoreeNo ratings yet
- 1.6. Introduction To Organic ChemistryDocument16 pages1.6. Introduction To Organic ChemistryRXNOFCHMNo ratings yet
- The IsomeriaDocument43 pagesThe IsomeriaScribdTranslationsNo ratings yet
- Quiz 2 Chem 30B S23Document2 pagesQuiz 2 Chem 30B S239kv5gc8sdtNo ratings yet
- CHM 102Document4 pagesCHM 102Fumzy AdelakunNo ratings yet
- Attachment 1 - 2024-02-21T191802.458Document15 pagesAttachment 1 - 2024-02-21T191802.458copadmmmmNo ratings yet
- CMY 284 Final Exam 2018Document11 pagesCMY 284 Final Exam 2018ntokozocecilia81No ratings yet
- Assignment NMR-spectrosDocument4 pagesAssignment NMR-spectrosShovan SwainNo ratings yet
- Qualitative NMR (#1) : Matt Prater Group 4 Analysis LabDocument1 pageQualitative NMR (#1) : Matt Prater Group 4 Analysis LabMatt PraterNo ratings yet
- CHEM 151 (Chapter 3)Document4 pagesCHEM 151 (Chapter 3)Chantel AceveroNo ratings yet
- Assignment Chapter 9 HydrocarbonsDocument1 pageAssignment Chapter 9 Hydrocarbonsaarushgoel025No ratings yet
- WM Revision SheetsDocument8 pagesWM Revision Sheetsdestine.ogieNo ratings yet
- Week 1 Study Groups Worksheet Bonding and Hybridisation: Exam PracticeDocument3 pagesWeek 1 Study Groups Worksheet Bonding and Hybridisation: Exam PracticeMehrNo ratings yet
- QBHi Ila 5 M ASc D0 H Z626Document2 pagesQBHi Ila 5 M ASc D0 H Z626Mathew WadeNo ratings yet
- Hachiya 2009Document3 pagesHachiya 2009ivanNo ratings yet
- Virtual Lab For Methyl OrangeDocument9 pagesVirtual Lab For Methyl OrangeKENT BENEDICT PERALESNo ratings yet
- Lec 3 Mass RevisionDocument3 pagesLec 3 Mass RevisionhaNo ratings yet
- Cuaderno de Trabajo - 2019-2Document35 pagesCuaderno de Trabajo - 2019-2Monica BravoNo ratings yet
- Structural ElucidationDocument6 pagesStructural Elucidationsimmysue003No ratings yet
- Assignment - AIA 2024Document4 pagesAssignment - AIA 2024KRITIKANo ratings yet
- Interpreting and Predicting Proton NMR Spectra - View As Single PageDocument64 pagesInterpreting and Predicting Proton NMR Spectra - View As Single PageMaxi MaNo ratings yet
- Chemistry Form Four ANSWER OF CHAPTER ONEDocument9 pagesChemistry Form Four ANSWER OF CHAPTER ONECali CaliNo ratings yet
- Check Carefully To Ensure You Are Sitting For The Correct PaperDocument9 pagesCheck Carefully To Ensure You Are Sitting For The Correct PaperfdfsdNo ratings yet
- Quantitative Biological and Clinical Mass Spectrometry: An IntroductionFrom EverandQuantitative Biological and Clinical Mass Spectrometry: An IntroductionNo ratings yet