Professional Documents
Culture Documents
02 AcidsSalts&Solubility
02 AcidsSalts&Solubility
Uploaded by
DiamondOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
02 AcidsSalts&Solubility
02 AcidsSalts&Solubility
Uploaded by
DiamondCopyright:
Available Formats
IGCSE Chemistry – Acids Alkalis Bases & Salts Revision Notes 2
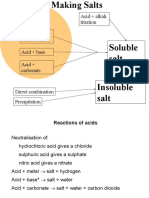
(4.5) Preparation of Salts – Reactions with Acids:
Learn these General Equations and their Examples:
1. ACID + METAL –––––––> SALT + HYDROGEN
Hydrochloric acid + Magnesium Magnesium Chloride + Hydrogen
2HCl(aq) + 2Mg(s) 2MgCl(aq) + H2(g)
Sulphuric acid + Zinc Zinc Sulphate + Hydrogen
H2SO4(aq) + Zn(s) ZnSO4(aq) + H2(g)
Nitric acid + Aluminium Aluminium Nitrate + Hydrogen
2HNO3(aq) + 2Al(s) 2AlNO3(aq) + H2(g)
2. ACID + BASE –––––––> SALT + WATER
Hydrochloric acid + Copper II Oxide Copper II Chloride + Water
HCl(aq) + CuO(s) CuCl(aq) + H2O(l)
Nitric acid + Aluminium Oxide Aluminium Nitrate + Water
6HNO3(aq) + Al2O3(aq) 2Al(NO3)3(aq) + 3H2O(l)
3. ACID + ALKALI –––––––> SALT + WATER
Sulphuric acid + Sodium Hydroxide Sodium Sulphate + Water
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l)
Phosphoric acid + Potassium Hydroxide Potassium Phosphate + Water
H3PO4(aq) + 3KOH(aq) K3PO4(aq) + 3H2O(l)
4. ACID + CARBONATE –––––––> SALT + WATER + CARBON DIOXIDE
Sulphuric acid + Ammonium Carbonate Ammonium Sulphate + Water + Carbon Dioxide
H2SO4(aq) + (NH4)2CO3(aq) (NH4)2SO4(aq) + H2O(l) + CO2(g)
Nitric acid + Sodium Carbonate Sodium Nitrate + Water + Carbon Dioxide
2HNO3 (aq) +Na2CO3(aq) 2NaNO3(aq) + H2O(l) + CO2(g)
Hydrochloric acid + Magnesium Carbonate Magnesium Chloride + Water + Carbon Dioxide
2HCl(aq)+ MgCO3(aq) MgCl2(aq) + H2O(l) + CO2(g)
(4.6) Solubility of Ionic Compounds – Learn the solubility patterns:
IGCSE Chemistry – Acids Alkalis Bases & Salts Revision Notes 2
(a) Soluble Compounds:
ALL: EXCEPT FOR:
(NO3+) Nitrate Salts (No Exceptions)
Sodium
(Na+)
Potassium
(K+) (No Exceptions
Salts Ammonium
(NH4+)
Chloride
(Cl-)
Bromide (Ag+)Silver
(Br-) (Halides)
Salts (Pb2+)Lead
(I-)
Iodide
Barium
(Ba2+)
Lead
(SO42-) Sulphate Salts (Pb2+) Sulphates
Calcium
(Ca2+)
(b) Insoluble Compounds:
ALL: EXCEPT FOR:
Sulphide Sodium
(S2-) (Na+) Sulphide
Carbonate Potassium
(CO32-) (K+) Carbonate
Salts Ammonium
(PO43-) (NH4+) Phosphate
Phosphate
Sodium
(Na+)
Potassium
(2.11- (OH-) Hydroxides (K+) Hydroxides 2.12)
Ammonium
(NH4+)
Hydrogen Chloride / Hydrochloric Acid: The Fountain Experiment:
Hydrogen Chloride is a colourless gas, denser than air – collected by
downward delivery
It is very highly soluble in water – a polar molecule – as it dissociates into
H+ and Cl- ions – cannot be collected over water.
This is an exothermic reaction which produces Hydrochloric Acid
Solution – with all the properties of an acid.
HCl(g) + H2O(l) HCl(aq) + H+ + Cl-
Its extreme solubility can be demonstrated in the ‘fountain experiment’ –
all the HCl gas in the round flask dissolves immediately in the first few
drops of water causing a vacuum – external air pressure forces more water
into the flask – universal indicator turns red to indicate acid is formed.
Hydrogen Chloride is also soluble in methylbenzene (toluene) BUT it does
not form an acidic solution – there is no dissociation – HCl stays in
molecular state – provided there is not a trace of water present.
Ammonia and Sulphur Dioxide gases are also highly soluble in water and will also perform the
‘fountain experiment’ – forming an alkaline and an acidic solution (of ions) respectively.
You might also like
- Bộ 200 câu hỏi mẫu & đáp án - EnglishScore PDFDocument147 pagesBộ 200 câu hỏi mẫu & đáp án - EnglishScore PDFPhạm Thị Bích Loan84% (19)
- CDR-EL-1109 - CDR Sample ElectricalDocument1 pageCDR-EL-1109 - CDR Sample ElectricalCDR Download100% (1)
- Garam Bab 8Document29 pagesGaram Bab 8ctohNo ratings yet
- SAlt Preperation - 1Document14 pagesSAlt Preperation - 1youssefelassal2009No ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Zapamti - Kiseline, Lu Ine, SoliDocument2 pagesZapamti - Kiseline, Lu Ine, SolidragoNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Electrolysis of Aqueoues SolutionDocument1 pageElectrolysis of Aqueoues SolutionSharonNo ratings yet
- Chapter 8: Salts: Flow Chart of Preparation of SaltsDocument7 pagesChapter 8: Salts: Flow Chart of Preparation of SaltsPrincess Ting TingNo ratings yet
- 4th Form Qualitative Analysis Sheet Summary SheetDocument2 pages4th Form Qualitative Analysis Sheet Summary SheetFrank MassiahNo ratings yet
- Chemistry, C8A - Aanotes (S)Document26 pagesChemistry, C8A - Aanotes (S)Farah Aisyah AhmadNo ratings yet
- Data Sheet Revision PDFDocument2 pagesData Sheet Revision PDFShifa RizwanNo ratings yet
- Topic7-Oxides and Salts-L2Document43 pagesTopic7-Oxides and Salts-L2haotongxu14No ratings yet
- 8A Salts - AnswerDocument14 pages8A Salts - AnswerWong Wai LunNo ratings yet
- Equations Involving AcidsDocument1 pageEquations Involving AcidsYoussef AmrNo ratings yet
- Chemistry Lab 16Document3 pagesChemistry Lab 16Nathaniel MorrisonNo ratings yet
- Predicting Reaction Products and NamingDocument2 pagesPredicting Reaction Products and Namingbistakenx2No ratings yet
- Acids, Bases and SaltsDocument4 pagesAcids, Bases and Saltsbubutrain2003No ratings yet
- Nomenclature: General Chemistry Pro-KnowledgeDocument2 pagesNomenclature: General Chemistry Pro-KnowledgemohammedNo ratings yet
- Periodic TableDocument2 pagesPeriodic TableAlliyah vidanesNo ratings yet
- Acids and Bases SummaryDocument2 pagesAcids and Bases SummaryTan Yong KhaiNo ratings yet
- Form 2 Introduction To SaltsDocument11 pagesForm 2 Introduction To Saltsemilykwamboka500No ratings yet
- Final Revision Acids, Bases and Salts (Repaired) PDFDocument13 pagesFinal Revision Acids, Bases and Salts (Repaired) PDFRawan Abd ElaatyNo ratings yet
- Part IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisDocument12 pagesPart IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisCharmine HolmesNo ratings yet
- 3 Experiment ChemistryDocument30 pages3 Experiment ChemistryThangavel SarujanNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- Cha 11Document11 pagesCha 11Tun Lin AungNo ratings yet
- OXIDES (Metals & Non-Metals)Document4 pagesOXIDES (Metals & Non-Metals)gauri guptaNo ratings yet
- Stuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListDocument2 pagesStuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListedeceNo ratings yet
- CA DEM Y: Chapter - 2 - NOTESDocument2 pagesCA DEM Y: Chapter - 2 - NOTESvarun puriNo ratings yet
- Form 4 Acid, Bases and Salts NotesDocument21 pagesForm 4 Acid, Bases and Salts NotesTamisha JacobsNo ratings yet
- Chemical ReactionsDocument2 pagesChemical ReactionsKrydztom UyNo ratings yet
- Csec Identification of Cations and AnionsDocument6 pagesCsec Identification of Cations and AnionsDarrion BruceNo ratings yet
- Chemical Reactions: Processes and TypesDocument38 pagesChemical Reactions: Processes and TypesSlay SacedaNo ratings yet
- Preparing Common Salts G8Document20 pagesPreparing Common Salts G8diamehta1512No ratings yet
- Types of OxideDocument1 pageTypes of OxideTamoya ShirleyNo ratings yet
- တက္ကသိုလ်ဝင်တန်း ဓာတုဗေဒ Dr.Soe Kyaw KyawDocument322 pagesတက္ကသိုလ်ဝင်တန်း ဓာတုဗေဒ Dr.Soe Kyaw KyawKhin OosweNo ratings yet
- 10th OswaalDocument24 pages10th OswaalAbhishek DwivediNo ratings yet
- Chemical Changes Mastery Part 4: AcidsDocument2 pagesChemical Changes Mastery Part 4: AcidsJoeNo ratings yet
- PowerPoint 2 Acids and MetalsDocument8 pagesPowerPoint 2 Acids and MetalsCT ONo ratings yet
- Edited - Acids Bases (Part 2) 6092 TeacherDocument27 pagesEdited - Acids Bases (Part 2) 6092 TeachersamrobbiesingcabarreraNo ratings yet
- PP Acid ReactionsDocument14 pagesPP Acid Reactionsapi-3696266No ratings yet
- !chemistry Review ANSDocument3 pages!chemistry Review ANSAngel LiNo ratings yet
- Appendix 1: The Periodic Table of The ElementsDocument7 pagesAppendix 1: The Periodic Table of The ElementshassanNo ratings yet
- Balancing Word Equations PracticeDocument3 pagesBalancing Word Equations PracticemmNo ratings yet
- Must Know For Chapter 9 - Salts (And C11 Qualitative Analysis)Document4 pagesMust Know For Chapter 9 - Salts (And C11 Qualitative Analysis)Chaw Wei HengNo ratings yet
- Acid Bases SummaryDocument8 pagesAcid Bases Summaryibraheemgamer786No ratings yet
- Standard Reduction Potential TablesDocument1 pageStandard Reduction Potential TablesJenver NanquiladaNo ratings yet
- Lesson Plan 5Document15 pagesLesson Plan 5Gusty DyanoNo ratings yet
- Solution 805196Document4 pagesSolution 805196scNo ratings yet
- Basic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairDocument33 pagesBasic Inorganic Chemistry PHR 125: Prof. Dr. Mona BedairAvvari AnnamaniNo ratings yet
- Salt AnalysisDocument6 pagesSalt Analysisashraf_mphilNo ratings yet
- Acids RecallDocument4 pagesAcids RecallAssumpta McguckinNo ratings yet
- Detection of Basic Radical Group 5, 6Document10 pagesDetection of Basic Radical Group 5, 6Abdul wahabNo ratings yet
- Solubility RulesDocument1 pageSolubility RulesAdamNo ratings yet
- Chemistry F4 SaltsDocument13 pagesChemistry F4 Saltscivichitam18No ratings yet
- Valency SheetDocument3 pagesValency SheetBex JacobsNo ratings yet
- Hydro Hydro Hydro: + Nonmetal+ Ic + Acid Nonmetal + Ic + Acid Nonmetal + + AcidDocument21 pagesHydro Hydro Hydro: + Nonmetal+ Ic + Acid Nonmetal + Ic + Acid Nonmetal + + AcidHani TamimiNo ratings yet
- Chap 02cDocument10 pagesChap 02cRCNo ratings yet
- 19 Jan S Block 2Document17 pages19 Jan S Block 2sachin anuseNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Vmware Vsphere - Pricing White Paper PDFDocument12 pagesVmware Vsphere - Pricing White Paper PDFsabeelshakirNo ratings yet
- COMPUTER ARCHITECTURE AND ORGANIZATION 11th Feb 2024Document4 pagesCOMPUTER ARCHITECTURE AND ORGANIZATION 11th Feb 2024Ddamulira CharlesNo ratings yet
- Roll No. Name PPO/PPI/Finals Final CompanyDocument48 pagesRoll No. Name PPO/PPI/Finals Final CompanyYash AgarwalNo ratings yet
- Lesson Plan, Refliction, Observation (Pattern)Document12 pagesLesson Plan, Refliction, Observation (Pattern)Hessa MohammedNo ratings yet
- BOSCH Lbb4432 KeypadDocument2 pagesBOSCH Lbb4432 KeypadJulie HalimNo ratings yet
- Intercultural and Global Communication ReviewerDocument4 pagesIntercultural and Global Communication ReviewerJESTON AMBUNANNo ratings yet
- Electronic Immobilizers For The Automotive Industry: U2270B Application NoteDocument19 pagesElectronic Immobilizers For The Automotive Industry: U2270B Application NoteRuslan ValiakhmetovNo ratings yet
- EMC Networker - Setting Up QoreStor As A CIFS, NFS Target On Dell EMC NetworkerDocument36 pagesEMC Networker - Setting Up QoreStor As A CIFS, NFS Target On Dell EMC NetworkerAziz BezzafNo ratings yet
- WB4 Short Form - Dual Branding - 10-24-19Document2 pagesWB4 Short Form - Dual Branding - 10-24-19joseNo ratings yet
- Single Award End of Feb 2023Document8 pagesSingle Award End of Feb 2023Lone G. MokgalagadiNo ratings yet
- Bernard Stiegler - Nanomutations, Hypomnemata and GrammatisationiDocument12 pagesBernard Stiegler - Nanomutations, Hypomnemata and GrammatisationiIoana AlbertNo ratings yet
- Chassis EUROCASE ML-5410 Middle Tower Full ATX Mainboard Supported, 7 Slots, PSU InsDocument5 pagesChassis EUROCASE ML-5410 Middle Tower Full ATX Mainboard Supported, 7 Slots, PSU InsGuran MaricioNo ratings yet
- Assam - WikipediaDocument32 pagesAssam - WikipediaNazrul IslamNo ratings yet
- MP2307 r1.9 PDFDocument12 pagesMP2307 r1.9 PDFNaciConSolNo ratings yet
- Retail Management Final ExamDocument17 pagesRetail Management Final ExamAkhilesh JadhavNo ratings yet
- CDP Proposal KavarattiDocument11 pagesCDP Proposal KavarattiShivansh Singh GautamNo ratings yet
- 1117 33Document8 pages1117 33tm5u2rNo ratings yet
- Excipient PT FinalDocument14 pagesExcipient PT FinalPreeti Wavikar-Panhale100% (1)
- Unit 4 Problems On Combined Bending and TorsionDocument8 pagesUnit 4 Problems On Combined Bending and TorsionAnonymous mRBbdopMKf100% (1)
- 3 Object Oriented Modeling and DesignDocument9 pages3 Object Oriented Modeling and DesignMaya M SNo ratings yet
- Retune For Live v1.4 READMEDocument5 pagesRetune For Live v1.4 README항가No ratings yet
- Republic Act No 3931 National Water and Air Pollution ControlDocument8 pagesRepublic Act No 3931 National Water and Air Pollution ControlKabayanNo ratings yet
- MAON Google SlidesDocument74 pagesMAON Google SlidesavinteumfilmsNo ratings yet
- History of Sanskrit Language and Kerala Culture McqsDocument8 pagesHistory of Sanskrit Language and Kerala Culture Mcqsmuzu136055No ratings yet
- Secrets of The Abundant Life SermonDocument4 pagesSecrets of The Abundant Life SermonJohnmarc Prince Montano100% (2)
- Minimum Sample For Diagnostic TestDocument6 pagesMinimum Sample For Diagnostic TestLulu DjalilNo ratings yet
- The Massive Korean Wave in Indonesia and Its Effects in The Term of CultureDocument6 pagesThe Massive Korean Wave in Indonesia and Its Effects in The Term of CultureRaven RhythmNo ratings yet
- BiotedadNo 3Document2 pagesBiotedadNo 3Budok MercadoNo ratings yet