Professional Documents
Culture Documents
Sdfsdfsfs
Sdfsdfsfs
Uploaded by
mwah mwahOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sdfsdfsfs
Sdfsdfsfs
Uploaded by
mwah mwahCopyright:
Available Formats
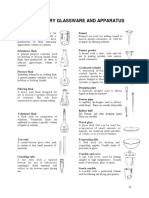
LABORATORY APPARATUS VOLUMETRIC FLASK
for measuring volume in
ERLENMEYER FLASKS the preparation of
used to contain liquids solutions and holds a
and for mixing, heating, precise amount of liquid
cooling, incubation, material when at room
filtration, storage, and temperature.
other liquid-handling
processes. have long necks with a
fill line on the neck for
their slanted sides and accurate measure.
narrow necks allow the
contents to be mixed by the lower area is a bulb
swirling without the risk shape with a flat
of spills, which is useful bottom for sitting on a
for titrations and for table or burner.
boiling liquids.
LIEBIG CONDENSER
has a broad flat bottom, used to condense
a conical body, and a vapors that pass
high cylindrical neck. through the inner glass
tube & It is cooled
FLORENCE FLASK down with water that
has a round body, a passes in the outer tube
long neck, and often a at the opposite
flat bottom. direction to the hot
vapors.
designed for uniform
heating, boiling, consists of a straight
distillation and ease of glass tube through
swirling. which the gas travels
and a water jacke which
DISTILLATION FLASK surrounds the tube and
used for distillation, the helps to cool the gas.
process of separating a
mixture of liquids with SEPARATORY FUNNELS
different boiling points used in the lab for
through evaporation liquid-liquid extractions,
and condensation. separating a mixture's
components into two
round-bottomed for solvent phases of
uniform heat different densities.
distribution, with a
stoppered or jointed usually in the shape of a
neck of variable length, globe or cylinder
to which is attached a provided with a
downward-sloping, stopcock for drawing off
inclined side-arm for the lower layer of a
connection to a mixture of immiscible
condensing unit or liquids.
similar component to
cool the heated vapor’s BURETTE
back down to their is used to dispense
liquid state. small volumes of liquid
called aliquots, or
sometimes gas, with
high accuracy.
consists of a long glass come in various sizes
tube with a valve at one and are shaped like a
end to control the flow cylinder.
of liquid.
GRADUATED CYLINDER
CENTRIFUGE TUBES has a narrow cylindrical
used to contain liquids shape with each
during centrifugation, marked line showing
which separates the the volume of liquid
sample into its being measured.
components by rapidly
rotating it around a while they are generally
fixed axis. more accurate and
precise than laboratory
have conical bottoms, flasks and beakers, they
which help collect any should not be used to
solid or heavier parts of perform volumetric
the sample being analysis.
centrifuged.
VOLUMETRIC PIPETTES
TEST TUBE transfer a single,
also known as a culture predetermined volume
tube or sample tube. of liquid.
a common piece of often called a bulb
laboratory glassware pipette for their shape,
consisting of a finger- which is a long tube-like
like length of glass or shaft at the bottom and
clear plastic tubing, top and a bulb in the
open at the top and center where the bulk
closed at the bottom. of solution is held.
TEST TUBE RACK PASTEUR PIPETTES
used to hold upright also known as droppers
multiple test tubes at or eye droppers, are
the same time. used to transfer small
quantities of liquids.
most commonly used
when various different usually glass tubes
solutions are needed to tapered to a narrow
work with point, and fitted with a
simultaneously, for rubber bulb at the top.
safety reasons, for safe
storage of test tubes,
and to ease the SEROLOGICAL PIPETTE
transport of multiple transfers liquids
tubes. measured in volume by
ml. Most have
Beaker graduations on the side
a glass container with a for measuring the liquid
flat bottom and a small being dispensed or
spout for pouring. aspirated.
is used in the chemistry have an edged color-
lab for mixing, heating, coded band that is used
and stirring liquids. for identification.
evaporation of the
MICROSPATULAS solvent.
Thin, pointed ends help
to transfer materials to ELECTRONIC BALANCE
small tubes or instrument used in the
microplates. accurate measurement
of weight of materials.
trough-shaped
spatulas: provides digital result of
Usually have one measurement
rounded and one
pointed end; can be MEASUREMENT IN CHEMISTRY
used to dislodge or
loosen materials in a DEFINITION OF TERMS
container. MEASUREMENT
the system or act of measuring comparison
pH METER of an unknown quantity with a known
an instrument used to
measure hydrogen ion ACCURACY
activity in solutions the closeness of a result to the true value.
(acidity/alkalinity).
PRECISION
consists of a glass the extent to which results agree with one
electrode and a read another
out screen.
a measure of consistency
CENTRIFUGE REPEATABILITY
used to separate referring to the consistency between
particles suspended in a individual values amongst a set of replicate
liquid according to measurements performed by the same
particle size and person at the same time on the same
density, viscosity of the sample, using the same method
medium, and rotor
speed. REPRODUCIBILITY
referring to the consistency of a method as
rotor driven instrument. used by different analysts, laboratories,
and/or over an extended time period
HOT PLATES STANDARD UNIT
laboratory tools used the units we usually use to measure the
to uniformly heat weight, length or capacity of objects.
samples.
MASS
are conceptually simple the amount/quantity of matter that an
– a flat surface with object possesses
heating elements. They
do not produce open WEIGHT
flames. A measure of the earth's gravitational attraction
for a body.
EVAPORATING DISH
a shallow usually lipped Density
vessel often of the mass of an object divided by its volume
porcelain used
especially for
concentrating solutions
on a small scale by SPECIFIC GRAVITY
the ratio of the density of one substance to is the clear liquid that lies above the solid
the density of another substance taken as a residue after centrifugation, precipitation,
standard crystallization or settling.
VOLUME CENTRIFUGATION
the amount of space occupied by matter. a method of separating molecules having
different densities by spinning them in
TEMPERATURE solution around an axis (in a centrifuge
a measure of the intensity of heat, or how rotor) at high speed.
hot a system is, regardless of its size.
CENTRIFUGATE
Metric System/International System rotate at very high speed in order to
a decimal system of units for separate the liquids from the solids
measurements of mass, length, time, and
other physical quantities Laboratory apparatus used to transfer liquid
from one container to another container:
ACT NO.4
PRECIPITATION PASTEUR PIPETTES (OR PIPETS)
the process of conversion of a solution into most commonly used tool for transferring
solid by converting the substance into small volumes of liquids from one container
insoluble form or by making the solution a to another.
super saturated one.
SEROLOGICAL PIPETTE
PRECIPITATES a type of sterile pipette that is used mainly
insoluble ionic solid products of a reaction, for cell culture and/or work with sterile
formed when certain cations and anions solutions
combine in an aqueous solution
called a terminal pipette, since the
FILTRATION graduations occupy the entire surface of
the process in which solid particles in a the pipette, including the tip
liquid or gaseous fluid are removed by the
use of a filter medium that permits the fluid VOLUMETRIC PIPETTES
to pass through but retains the solid calibrated to deliver a certain volume of a
particles solution free drainage
FILTRATE bulb pipettes or belly pipettes
a liquid that has passed through the
filtration process Proper handling of glassware can reduce the
risk of injury and accident:
DECANTATION
the process of separation of liquid from Never carry a flask by its neck.
solid and other immiscible (non-mixing)
liquids, by removing the liquid layer at the Never carry a beaker by its side.
top from the layer of solid or liquid below
Always use two hands carrying any
DECANTATE glassware (position one hand under the
the liquid component that is decanted glass for support) Appropriate glove should
be worn whe there is a risk of breakage (eg
RESIDUE inserting a glass rod), chemical
the part that is left after the main part has contamination, or thermal hazard
gone or been taken away, or a substance
that remains after a chemical process such Most common method to clean glassware:
as evaporation
Rinse the glassware with the appropriate
solvent Use deionized water for water-
soluble contents
SUPERNATANT
Use ethanol for ethanol-soluble contents,
followed by
rinses in deionized water • Rinse with other
solvents as needed, followed by ethanol,
and finally deionized water
If the glassware requires scrubbing, scrub
with a brush using hot soapy water rinse
thoroughly with tap water, followed by
rinses with deionized water
Washing with soap and water:
Soak the glassware in soap solution for at
least 10 to 15 minutes or leave overnight.
Scrub with a brush or cloth or sponge if
needed Rinse thoroughly with tap water
Again rinse with distilled or deionized water
If you need this glassware soon, then rinse
with acetone or ethanol
Common Methods of Separating a Solid-Liquid
Mixture.
Chromatography
a separation technique based on how the
different components in a mixture have
different affinity for the stationary and
mobile phase
DISTILLATION
a separation technique used to separate
components of a liquid mixture by a
process of heating and cooling
EVAPORATION
a separation method used to separate of a
mixture of a liquid with a dissolved solid,
involving removal of a liquid evaporating it
and leaving behind a solid
NITRATION
a separation technique used to separate
the components of a mixture containing an
undissolved sold in a liquid by using a
funnel lined with fer paper to retain the
solids while letting the liquid through
You might also like
- Opel GM Crossland X 2019 Wiring DiagramsDocument22 pagesOpel GM Crossland X 2019 Wiring Diagramsdaniellelane200586msd100% (131)
- A Guide to Perfume Production - A Selection of Vintage Articles on the Methods and Ingredients of PerfumeryFrom EverandA Guide to Perfume Production - A Selection of Vintage Articles on the Methods and Ingredients of PerfumeryRating: 5 out of 5 stars5/5 (2)
- Advanced Trauma Life Support (Atls)Document44 pagesAdvanced Trauma Life Support (Atls)Danar Syahrial PradhiptaNo ratings yet
- Acdc1000 11124 & 11226Document34 pagesAcdc1000 11124 & 11226Jaime ArreolaNo ratings yet
- Laboratory Glassware and Apparatus LSMDocument4 pagesLaboratory Glassware and Apparatus LSMShiina Mashiro100% (1)
- Revised GCL I Lab ManualDocument95 pagesRevised GCL I Lab ManualomskirtNo ratings yet
- LAS 1 Lab Apparatus and MaterialsDocument50 pagesLAS 1 Lab Apparatus and MaterialsZeian Jacob BaylaNo ratings yet
- Laboratory ApparatusDocument10 pagesLaboratory ApparatusSEAN GAVIN DELOS REYESNo ratings yet
- Common Laboratory ApparatusesDocument2 pagesCommon Laboratory ApparatusesAlowee AbelloNo ratings yet
- Laboratory Activity #2Document6 pagesLaboratory Activity #2JONES RHEY KIPASNo ratings yet
- Common Lab Apparatus and ProcedureDocument12 pagesCommon Lab Apparatus and ProcedureRammohan Balaji PrasadNo ratings yet
- GenchemDocument2 pagesGenchemChristian MorfeNo ratings yet
- CHE1L-Exp Act - 1Document6 pagesCHE1L-Exp Act - 1Kayezel SinohinNo ratings yet
- Common Laboratory EquipmentDocument2 pagesCommon Laboratory EquipmentYuan BrionesNo ratings yet
- Activity 1Document4 pagesActivity 1Hannah VisitacionNo ratings yet
- Lab Apparatus FinalDocument23 pagesLab Apparatus Finalshrf btuanNo ratings yet
- Phar 205Document156 pagesPhar 205billhaddNo ratings yet
- Common Laboratory EquipmentDocument50 pagesCommon Laboratory EquipmentChristian Josh EspedillonNo ratings yet
- Basic Laboratory ApparatusDocument12 pagesBasic Laboratory Apparatusrose ann claveriaNo ratings yet
- CHEM Midterm NotesDocument7 pagesCHEM Midterm NotesKharylle Ann IglesiasNo ratings yet
- Chemistry ApparatusDocument3 pagesChemistry ApparatusMylz MendozaNo ratings yet
- Laboratory HW1Document2 pagesLaboratory HW1lizaNo ratings yet
- Common Laboratory ApparatusDocument21 pagesCommon Laboratory ApparatusMaria Kristelle Alejandro PaltaoNo ratings yet
- Bio 024 Lab Activity 1 Lab ApparatusesDocument6 pagesBio 024 Lab Activity 1 Lab ApparatusesLydin Ayson AquinoNo ratings yet
- SolutionsDocument21 pagesSolutionsmuhammad asim shaikhNo ratings yet
- Evaporation and ExtrusionDocument16 pagesEvaporation and ExtrusionSharmaine RoseNo ratings yet
- Experiment 1 - Laboratory Apparatus 1Document6 pagesExperiment 1 - Laboratory Apparatus 1Raymart DomingoNo ratings yet
- Dissilation Apparatus Uses and PartsDocument5 pagesDissilation Apparatus Uses and PartsMa. Lilian Jem MonteroNo ratings yet
- Equipment in Chemistry LaboratoryDocument5 pagesEquipment in Chemistry LaboratoryIstiva AmeiliaNo ratings yet
- Common Laboratory EquipmentDocument16 pagesCommon Laboratory Equipmentbonbonreyes9No ratings yet
- Bio 024 Lab Activity 1 - Lab ApparatusesDocument7 pagesBio 024 Lab Activity 1 - Lab ApparatusesLydin Ayson AquinoNo ratings yet
- 50 Laboratory Apparatuses Biochemistry: OCTOBER 31, 2020Document14 pages50 Laboratory Apparatuses Biochemistry: OCTOBER 31, 2020Allen TandocNo ratings yet
- Basic Chemistry Apparatus AaaaDocument4 pagesBasic Chemistry Apparatus Aaaahoranlovely5No ratings yet
- Et ST 9 Mod - 1Document16 pagesEt ST 9 Mod - 1pdchemigcse1786No ratings yet
- Lab ShitDocument4 pagesLab ShitpidoghislaineNo ratings yet
- Laboratory ApparatusDocument76 pagesLaboratory ApparatusNikki EbilloNo ratings yet
- Laboratory ApparatusDocument18 pagesLaboratory ApparatusCyrus De LeonNo ratings yet
- Lab 4Document24 pagesLab 44n6hg5h7hgNo ratings yet
- Chemistry ApparatusDocument7 pagesChemistry ApparatusGalino Julia Cristine A.No ratings yet
- BS MTDocument3 pagesBS MTGenrev LisondraNo ratings yet
- Laboratory ApparatusDocument48 pagesLaboratory ApparatusYixue WangNo ratings yet
- BiochemDocument7 pagesBiochemjolibeecaldonaNo ratings yet
- Group 3Document11 pagesGroup 3Kyle's ChannelNo ratings yet
- Laboratory ApparatusDocument2 pagesLaboratory ApparatuskdvillanuevaNo ratings yet
- Common Apparatus and ProceduresDocument7 pagesCommon Apparatus and ProceduresNorazrina Abdul Aziz0% (1)
- Activity No 1Document9 pagesActivity No 1Mary Cuba GarciaNo ratings yet
- Common Laboratory GlasswareDocument3 pagesCommon Laboratory GlasswareRegina SantosNo ratings yet
- Laboratory EquipmentsDocument5 pagesLaboratory EquipmentsMyca Katrina CantaneroNo ratings yet
- Worksheet 1 For Laboratory Glasswares and Equipment 2Document11 pagesWorksheet 1 For Laboratory Glasswares and Equipment 2Akeysha CarreonNo ratings yet
- Laboratory Apparatus and Their FunctionsDocument7 pagesLaboratory Apparatus and Their FunctionsLinzieeNo ratings yet
- Laboratory ApparatusDocument3 pagesLaboratory ApparatusRacky Carag67% (3)
- Laboratory ApparatusDocument17 pagesLaboratory Apparatuszena100% (1)
- Assignment: Inorganic and Organic Chemistry CHM101Document3 pagesAssignment: Inorganic and Organic Chemistry CHM101Cj DelfinNo ratings yet
- Apparatus Description UsesDocument6 pagesApparatus Description UsesVenus De GraciaNo ratings yet
- Lab ApparatusDocument10 pagesLab ApparatuslizNo ratings yet
- Chemistry Laboratory: Group 1Document51 pagesChemistry Laboratory: Group 1Rachel Ann De LeonNo ratings yet
- Laboratory ApparatusDocument4 pagesLaboratory ApparatusBugayong Manzon BethNo ratings yet
- Basuel Exercise 1 Lab ApparatusDocument16 pagesBasuel Exercise 1 Lab ApparatusOrdinary GuitaristNo ratings yet
- Stress-Free Science: A Visual Guide to Acing Science in Grades 4-8From EverandStress-Free Science: A Visual Guide to Acing Science in Grades 4-8No ratings yet
- Section Cutting and Staining: A practical introduction to histological methods for students and practitionersFrom EverandSection Cutting and Staining: A practical introduction to histological methods for students and practitionersNo ratings yet
- Chemistry Lab Mysteries, Fun Laboratory Tools! Chemistry for Kids - Children's Analytic Chemistry BooksFrom EverandChemistry Lab Mysteries, Fun Laboratory Tools! Chemistry for Kids - Children's Analytic Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Soap Bubbles: Their Colors and Forces Which Mold ThemFrom EverandSoap Bubbles: Their Colors and Forces Which Mold ThemRating: 4.5 out of 5 stars4.5/5 (15)
- PRMLSP 1Document11 pagesPRMLSP 1mwah mwahNo ratings yet
- Aesthetic Desktop #1 Free - Copy (Autosaved)Document28 pagesAesthetic Desktop #1 Free - Copy (Autosaved)mwah mwahNo ratings yet
- Q2 Contemporary Arts ReviewerDocument2 pagesQ2 Contemporary Arts Reviewermwah mwahNo ratings yet
- Influence of Organic and Inorganic Fertilizers On Growth, Yield and Physio-Chemical Properties of PapayaDocument16 pagesInfluence of Organic and Inorganic Fertilizers On Growth, Yield and Physio-Chemical Properties of Papayamwah mwahNo ratings yet
- 1 PBDocument11 pages1 PBmwah mwahNo ratings yet
- Activity Sheet in Science (Specialized Subject-Stem) Grade Level Quarter / Domain Week & Day NO. LC CodeDocument6 pagesActivity Sheet in Science (Specialized Subject-Stem) Grade Level Quarter / Domain Week & Day NO. LC Codemwah mwahNo ratings yet
- WGVWTBRBTDocument19 pagesWGVWTBRBTmwah mwahNo ratings yet
- Q-PCF-12-30-PN 6Document1 pageQ-PCF-12-30-PN 6Camilo Maya PodestaNo ratings yet
- M/S. Sri Kanakadurga Tribal Labour Contract Mutually Aided Co-Op Society.Document11 pagesM/S. Sri Kanakadurga Tribal Labour Contract Mutually Aided Co-Op Society.Daneshwer VermaNo ratings yet
- My Business PlanDocument7 pagesMy Business Planadeoye sheyiNo ratings yet
- Semester Scheme Odd21Document90 pagesSemester Scheme Odd21Rahul SinghNo ratings yet
- Chain of InfectionDocument8 pagesChain of Infectionkim mimiNo ratings yet
- Lab ManualDocument51 pagesLab Manualvani_prkshNo ratings yet
- PhySciSHS Q4 Week1Document46 pagesPhySciSHS Q4 Week1Roseman TumaliuanNo ratings yet
- Soal Bhs Ing RafiDocument7 pagesSoal Bhs Ing RafidarmawanNo ratings yet
- 5 - NLM Practice Assignment @JEEAdvanced - 2024Document5 pages5 - NLM Practice Assignment @JEEAdvanced - 2024Vineet SierraNo ratings yet
- Marxism by Thozhar ThiyaguDocument4 pagesMarxism by Thozhar ThiyaguMathursathyaNo ratings yet
- Preparing For EnemaDocument11 pagesPreparing For Enemapassion26100% (1)
- Using Geophysics To Characterize The Subsurface: The PrinciplesDocument152 pagesUsing Geophysics To Characterize The Subsurface: The PrinciplesMasahiro Galang SusantoNo ratings yet
- # Sample Quotation Copy For Go Kart SparesDocument3 pages# Sample Quotation Copy For Go Kart SparessaqibNo ratings yet
- Orthogonally Stiffened Plates Hoppman W.H. 1953Document46 pagesOrthogonally Stiffened Plates Hoppman W.H. 1953Iliyah87No ratings yet
- Standard Moisture Regain and Moisture Content of FibersDocument3 pagesStandard Moisture Regain and Moisture Content of Fibersff fixNo ratings yet
- Apply Good Dispensing Principles - NEW-1Document49 pagesApply Good Dispensing Principles - NEW-1abelashe2No ratings yet
- AsDocument777 pagesAsCristhian QuimizNo ratings yet
- Providing Food Stations For Stray Animals: National Service Training ProgramDocument8 pagesProviding Food Stations For Stray Animals: National Service Training ProgramLeane ZapantaNo ratings yet
- Method 207 Ammonia in Air EmissionDocument49 pagesMethod 207 Ammonia in Air Emissionsalma ghaniNo ratings yet
- Factsheet - REPowerEU PDFDocument2 pagesFactsheet - REPowerEU PDFconor farrellNo ratings yet
- Foodsupplements 6-3-2019Document282 pagesFoodsupplements 6-3-2019TalalAlamoudiNo ratings yet
- Cradles of Early Science Development of Science in Asia IndiaDocument4 pagesCradles of Early Science Development of Science in Asia IndiaAvox EverdeenNo ratings yet
- DimondDocument1 pageDimondapi-318888783No ratings yet
- Compair L160Document72 pagesCompair L160Mohamed MusaNo ratings yet
- Iq TestDocument18 pagesIq TestAmrita BawaNo ratings yet
- Tenopa SC LabelDocument4 pagesTenopa SC LabelSavvas DimitriadisNo ratings yet
- Math Geometry VocabularyDocument21 pagesMath Geometry VocabularyLekshmi R VasundharanNo ratings yet