Professional Documents
Culture Documents
Optic Neuritis
Optic Neuritis
Uploaded by
jai.kumar.maingi0 ratings0% found this document useful (0 votes)
4 views13 pagesCopyright
© © All Rights Reserved
Available Formats
XLSX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
0 ratings0% found this document useful (0 votes)
4 views13 pagesOptic Neuritis
Optic Neuritis
Uploaded by
jai.kumar.maingiCopyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
You are on page 1of 13
Rank NCT NumbTitle Acronym Status Study ResuConditionsInterventioOutcome MSponsor/Co
1 NCT017211BIIB033 In Acute OptiCompletedHas Result Acute OpticBiological Change in Biogen
2 NCT047620OCS-05 in PACUITY Recruiting No Results Optic NeuriDrug: OCS-Number ofOculis|Neur
p
3 NCT008566A Randomized, Double-b CompletedHas Result Optic NeuriDrug: Glat Retinal Ne Teva Brand
4 NCT019871Defining thACHTAR CompletedNo Results Optic NeuriDrug: ACT Neuro-prote Neuro-Opht
5 NCT036304BN201 SAD MAD Study CompletedNo Results Optic NeuriDrug: CompSafety: Ad Accure The
6 NCT013379Ampyra for Optic Neuri CompletedHas Result Multiple ScDrug: Dalf Efficacy of Washington
7 NCT026579Long-TermRENEWED CompletedHas Result Acute OpticDrug: Plac FF-VEP Lat Biogen
8 NCT028331Pharmacokinetics, SafCompletedNo Results Multiple ScDrug: BIIB PK paramete Biogen
9 NCT001794Long Term CHAMPION
S CompletedHas Result Multiple S Drug: inte Rate of De Beth Israe
10 NCT005017Safety and Tolerabilit CompletedNo Results NeuromyeliDrug: Ritu Safety and University
11 NCT017774Efficacy of BevacizumCompletedHas Result NeuromyeliDrug: BevaBaseline ExJohns Hopk
12 NCT042012An Efficacy and Safet Active, notHas Result NeuromyeliBiological: Number ofAlexion
13 NCT041554A Study of the Safety Active, notNo Results NeuromyeliDrug: Ecul Change FroAlexion
14 NCT020031An Open Label ExtensiCompletedHas Result NeuromyeliBiological: Number ofAlexion
15 NCT020732Efficacy and Safety S CompletedHas Result NeuromyelDrug: Satr Time to Fi Hoffmann-L
16 NCT020288Efficacy and Safety CompletedHas Result NeuromyelDrug: Satr Time to Fi Hoffmann-L
17 NCT051453Clinical Study of B00 Recruiting No Results NMO SpectDrug: B001Dose-limit Shanghai P
18 NCT046707An Open Label Study CompletedNo Results NeuromyeliDrug: DrugEvaluate thReistone B
19 NCT057306Efficacy anAQUARELLRecruiting No Results NeuromyeliBiological: Time to theBiocad
20 NCT055515Efficacy and Safety o Not yet recNo Results NeuromyeliDrug: MitoTime to FirCSPC Zhong
21 NCT055492Study of InebilizumabRecruiting No Results NeuromyeliDrug: Ineb Maximum Obs Horizon The
22 NCT053463Efficacy and Safety S Recruiting No Results NeuromyeliDrug: RavuChange FroAlexion
23 NCT053140A Study of MIL62 in Recruiting No Results NeuromyeliDrug: MIL6Phase 1b: Beijing Mab
24 NCT052696A Study In SAkuraBonsRecruiting No Results NeuromyeliDrug: Satr ProportionHoffmann-L
25 NCT051996A Study ToSAkuraSunRecruiting No Results NeuromyeliDrug: Satr Summary of Hoffmann-
26 NCT046605A Study to Evaluate t Active, notNo Results NeuromyeliDrug: satr PercentageHoffmann-
27 NCT046292In Vitro Study of the Recruiting No Results NeuromyeliProcedure:Measure inImcyse SA
28 NCT042274A Study of HBM9161 CompletedNo Results NMO SpectDrug: HBM9 Number ofHarbour Bi
29 NCT041462A Phase I Clinical Tri Recruiting No Results NeuromyeliDrug: BAT Dose-limit Bio-Thera S
30 NCT054327A Study of LP-168 in CompletedNo Results Multiple S Drug: LP-16Number ofGuangzhou
31 NCT033304A Phase III Study of Recruiting No Results NeuromyeliBiological: Time to fir RemeGen Co
32 NCT022007N-MOmentum: A Clinic CompletedHas Result NeuromyeliDrug: Ineb Time to AdMedImmun
33 NCT031384Central Pain Study fo CompletedNo Results Neuromyelit Drug: ABX-Change in m Abide Ther
34 NCT018455Phase II Clinical Tri CompletedNo Results NeuromyeliDrug: NPB-Change froNihon Phar
35 NCT058400ACT With NMOSD Patie Not yet recNo Results NMO SpectBehavioralChanges inThomas Jef
36 NCT059742A Phase IIbSENS-NMONot yet recNo Results NMO Spectr Drug: NabiChange in Michael, L
37 NCT003042A Pilot Study of Mito CompletedNo Results NeuromyeliDrug: MitoRelapse ra State Univ
38 NCT021663Safety and Efficacy o CompletedHas Result TransverseDrug: Dalf Walking SpJohns Hopk
39 NCT045615Safety and CARTinNS Recruiting No Results Autoimmune Biological Types and iTongji Hosp
40 NCT017596C1-esterase Inhibitor CompletedHas Result NeuromyeliDrug: C1-esNumber ofMichael Le
41 NCT009048An Open Label Study oCompletedHas Result NeuromyeliDrug: Ecul Median Num Mayo Clini
42 NCT010645Safety Study of a Sin CompletedNo Results Optic Atro Drug: QPI- To determin Quark Phar
43 NCT046269A Multi-CeVISIONMS-Active, notNo Results Relapsing M Drug: CNMChange in Clene Nano
44 NCT052841A Study of Orelabruti Not yet recNo Results NeuromyeliDrug: OrelaAnnualizedPeking Unio
45 NCT007725Single Oral Doses StudCompletedNo Results Multiple ScDrug: Neri Visual EvokSanofi
Gender Age Phases EnrollmentFunded ByStudy TypeStudy Desi Other IDs Start Date Primary Co
All 18 Years toPhase
55 Years
2 Â (Adult)82 Industry InterventioAllocation 215ON201 Dec-12 Oct-14
All 18 Years toPhase
60 Years
2 Â (Adult)42 Industry|OInterventioAllocation:OCS-05_P2February 1October 30
All 18 Years toPhase
45 Years
3 Â (Adult)44 Industry InterventioAllocation:PM030 Feb-09 Dec-10
All 18 Years and
Early
older
Phase
 (Adult, Older
25 Other|Indu
Adult) InterventioAllocation 13-330|AC Nov-13 Dec-16
All 18 Years toPhase
55 Years
1 Â (Adult)48 Industry InterventioAllocation BN201_RDR May 27, 20February 1
All 18 Years toPhase
55 Years
2|Ph (Adult)53 Other|InduInterventioAllocation Ampyra Vi May-11 Dec-13
All 18 Years and
Phase
older
2 Â (Adult, Older
52 Industry
Adult) InterventioAllocation 215ON203|March 10, January 23
All 18 Years toPhase
55 Years
1 Â (Adult)28 Industry InterventioAllocation:215HV103 Jul-16 Nov-16
All 18 Years and
Phase
older
4 Â (Adult,155
Older
Other|Indu
Adult) InterventioAllocation 2003P00008 Feb-01 Mar-09
All 12 Years toPhase
86 Years
1 Â (Child,20 Adult,
Other|Indu
Older Adult)
InterventioAllocation H7620-253 Jan-04 Dec-10
All 18 Years toPhase
70 Years
1 Â (Adult,10Older
Other|Indu
Adult) InterventioAllocation Avastin_N Jun-13 Feb-15
All 18 Years and
Phase
older
3 Â (Adult, Older
58 Industry
Adult) InterventioAllocation ALXN1210 December March 15,
All 2 Years to 17
Phase
Years
2|Ph
 (Child) 12 Industry InterventioAllocation ECU-NMO-3 January 14August 16,
All 18 Years and
Phase
older
3 Â (Adult,119
Older
Industry
Adult) InterventioAllocation ECU-NMO-3 January 12July 12, 20
All 18 Years toPhase
74 Years
3 Â (Adult,95Older
Industry
Adult) InterventioAllocation BN40900|SAugust 5, October 12
All 12 Years toPhase
74 Years
3 Â (Child,85 Adult,
Industry
Older Adult)
InterventioAllocation BN40898|2February 2June 6, 20
All 18 Years toEarly
70 Years
Phase (Adult,45Older
Industry
Adult) InterventioAllocation B001-103 April 7, 20 June 15, 2
All 18 Years toPhase
75 Years
2 Â (Adult,10Older
Industry
Adult) InterventioAllocation RSB20621 January 20August 15,
All 18 Years and
Phase
older
3 Â (Adult,105
Older
Industry
Adult) InterventioAllocation BCD-132-6December Sep-24
All 18 Years toPhase
60 Years
2 Â (Adult)45 Industry InterventioAllocation HE071-CSPNovember October 15
All 2 Years to 17
Phase
Years
2 Â (Child) 15 Industry InterventioAllocation VIB0551.P August 25,September
All 2 Years to 17
Phase
Years
2|Ph
 (Child) 12 Industry InterventioAllocation ALXN1210 June 23, 2 March 1, 2
All 18 Years toPhase
60 Years
3 Â (Adult)140 Industry InterventioAllocation MIL62-CT3August 18, Mar-25
All 18 Years toPhase
74 Years
4 Â (Adult,100Older
Industry|OInterventioAllocation
Adult) MN42928|2 August 2, May 29, 20
All 2 Years to 11
Phase
Years
3 Â (Child) 8 Industry InterventioAllocation WN41733|2 SeptemberSeptember
All 18 Years and
Phase
older
3 Â (Adult,119
Older
Industry
Adult) InterventioAllocation WN42349|2 March 2, 2May 25, 20
All 18 Years toNot
65 Years
Applic (Adult,30Older
Industry
Adult) InterventioAllocation IMCY-NMOJune 15, 2 February 1
All 18 Years and
Phase
older
1 Â (Adult, Older
9 Industry
Adult) InterventioAllocation 9161.2 March 31, December
All 18 Years toPhase
65 Years
1 Â (Adult,15Older
Industry
Adult) InterventioAllocation BAT-4406FSeptemberMarch 13,
All 18 Years toPhase
55 Years
1 Â (Adult)70 Industry InterventioAllocation LP-168-CN May 14, 20November
All 18 Years toPhase
65 Years
3 Â (Adult,166Older
Industry
Adult) InterventioAllocation C009NMOSJanuary 29 Oct-23
All 18 Years and
Phase
older
2|Ph
 (Adult,231
Older
Industry
Adult) InterventioAllocation CD-IA-MEDApril 1, 20 October 26
All 18 Years and
Phase
older
1 Â (Adult, Older
9 Industry
Adult) InterventioAllocation ABX-1431_August 1, July 23, 20
All 20 Years and
Phase
older
2 Â (Adult, Older
7 Industry
Adult) InterventioAllocation NPB-01-08 May-13 Aug-15
All 18 Years and
Notolder
Applic
 (Adult, Older
50 Other|Indu
Adult) InterventioAllocation iRISID-202 June 1, 20 December
All 18 Years and
Phase
older
2 Â (Adult, Older
44 Other|Indu
Adult) InterventioAllocation sens_nmo_ Apr-24 Apr-26
All 18 Years toPhase
55 Years
4 Â (Adult) 5 Other|InduInterventioAllocation JNI-NMO-1 Aug-01
All 18 Years toPhase
70 Years
2 Â (Adult,24Older
Other|Indu
Adult) InterventioAllocation NA_00090 Feb-14 January 8,
All 18 Years toEarly
75 Years
Phase (Adult,18Older
Other|Indu
Adult) InterventioAllocation CARTinNS SeptemberFebruary 2
All 18 Years toPhase
65 Years
1 Â (Adult,10Older
Other|Indu
Adult) InterventioAllocation NA_00078 Jan-13 Nov-13
All 18 Years and
Phase
older
1|Ph
 (Adult, Older
14 Other|Indu
Adult) InterventioAllocation 09-001240 Apr-09 Dec-11
All 50 Years and
Phase
older
1 Â (Adult, Older
48 Industry
Adult) InterventioAllocation QRK.007 Feb-10 Apr-13
All 18 Years toPhase
55 Years
2|Ph (Adult)
150 Industry|OInterventioAllocation CNMAu8.2October 22 Jun-24
All 18 Years toNot
75 Years
Applic (Adult,23Older
Other|Indu
Adult) InterventioAllocation ICP-022-00 Apr-22 Aug-23
All 18 Years and
Phase
older
2 Â (Adult, Older
31 Industry
Adult) InterventioAllocation ACT10573 Sep-08 Jun-09
CompletionFirst Poste Results Fir Last UpdatLocations Study Doc URL
Oct-14 November June 30, 2 June 30, 2 Research Site, Parkvi https://ClinicalTrials.gov/show/NCT01721161
March 31, February 21, 2021 May 11, 20Hospices Civils de Lyohttps://ClinicalTrials.gov/show/NCT04762017
Feb-11 March 6, 2SeptemberFebruary 6, 2018 https://ClinicalTrials.gov/show/NCT00856635
Dec-16 November 19, 2013 August 22,Neuro Ophthalmology, https://ClinicalTrials.gov/show/NCT01987167
February 2August 15, 2018 April 10, 2 Simbec Research Limithttps://ClinicalTrials.gov/show/NCT03630497
Dec-13 April 19, 2 January 30January 30Washington Universityhttps://ClinicalTrials.gov/show/NCT01337986
January 23January 18SeptemberSeptemberResearch S"Statistica https://ClinicalTrials.gov/show/NCT02657915
Nov-16 July 14, 2016 December Research Site, Madisohttps://ClinicalTrials.gov/show/NCT02833142
Mar-09 SeptemberSeptemberSeptemberMS Treatment Centerhttps://ClinicalTrials.gov/show/NCT00179478
a
Dec-10 July 16, 2007 SeptemberUCSF MS Center , 350https://ClinicalTrials.gov/show/NCT00501748
May-15 January 28May 29, 20August 21,Johns Hopkins Hospitahttps://ClinicalTrials.gov/show/NCT01777412
July 31, 20 December August 9, August 9, Clinical St "Study Prothttps://ClinicalTrials.gov/show/NCT04201262
July 31, 20 November 7, 2019 July 27, 20 Clinical Trial Site, Sa https://ClinicalTrials.gov/show/NCT04155424
July 12, 20 December August 23,August 23, Mayo Clinic"StudyArizona,
Prot
Scottsdale,
https://ClinicalTrials.gov/show/NCT02003144
Arizona, United States|The Research Center of Sou
January 31February 2December March 1, 2University "Study Prothttps://ClinicalTrials.gov/show/NCT02073279
December January 7, December April 18, 2 Children's "Study Prothttps://ClinicalTrials.gov/show/NCT02028884
December December 6, 2021 May 11, 20Beijing Tiantan Hospithttps://ClinicalTrials.gov/show/NCT05145361
August 15,December 17, 2020 March 23, Xiangya Hospital Of Chttps://ClinicalTrials.gov/show/NCT04670770
Apr-25 February 16, 2023 February 2Llc "Profimed", Barnahttps://ClinicalTrials.gov/show/NCT05730699
April 15, 2 September 22, 2022 September 22, 2022 https://ClinicalTrials.gov/show/NCT05551598
SeptemberSeptember 22, 2022 August 2, UC San Diego Altman https://ClinicalTrials.gov/show/NCT05549258
Clinical and Translational Research Institute (ACTRI) Building,
March 30, April 26, 2022 June 13, 2 Clinical Trial Site, M https://ClinicalTrials.gov/show/NCT05346354
Mar-27 April 6, 2022 August 14,Ethics Committee of Ch https://ClinicalTrials.gov/show/NCT05314010
January 15March 8, 2022 August 1, Stanford Health Care,https://ClinicalTrials.gov/show/NCT05269667
February 2January 20, 2022 August 7, Children's Hospital Colorado.,
https://ClinicalTrials.gov/show/NCT05199688
Denver, Colorado, United States|Hospital de Pediatr
May 25, 20December 9, 2020 July 14, 20 Children's Hospital o https://ClinicalTrials.gov/show/NCT04660539
February 1November 16, 2020 March 1, 2Hôpital Pierre Werthhttps://ClinicalTrials.gov/show/NCT04629274
December January 13, 2020 January 25Nanfang Hospital, Gu https://ClinicalTrials.gov/show/NCT04227470
June 1, 20 October 31, 2019 April 28, 2 Huashan Hospital affi https://ClinicalTrials.gov/show/NCT04146285
December June 27, 2022 February 2The Second Affiliated https://ClinicalTrials.gov/show/NCT05432713
Oct-23 November 6, 2017 January 11Beijing Hospital, Beiji https://ClinicalTrials.gov/show/NCT03330418
November July 25, 20 December December Research Site, "Study
Birmingham,
Prothttps://ClinicalTrials.gov/show/NCT02200770
Alabama, United States|Research Site, Sacramento, Ca
July 24, 20 May 3, 2017 January 25The Walton Centre, Lihttps://ClinicalTrials.gov/show/NCT03138421
Aug-15 May 3, 2013 April 12, 2 Japan, Osaka,, Japan https://ClinicalTrials.gov/show/NCT01845584
December May 3, 2023 May 3, 2023 https://ClinicalTrials.gov/show/NCT05840055
Sep-26 August 3, 2023 August 3, Massachusetts General https://ClinicalTrials.gov/show/NCT05974293
May-04 March 17, 2006 December Baird Multiple Scleroshttps://ClinicalTrials.gov/show/NCT00304291
January 8, June 18, 2 April 17, 2 April 17, 2 Johns Hopkins Univershttps://ClinicalTrials.gov/show/NCT02166346
May 31, 20September 23, 2020 April 12, 2 Tongji Hospital affil https://ClinicalTrials.gov/show/NCT04561557
Nov-13 January 3, July 18, 20 July 18, 20 Johns Hopkins Hospitahttps://ClinicalTrials.gov/show/NCT01759602
Dec-11 May 20, 20November November Mayo Clinic, Scottsda https://ClinicalTrials.gov/show/NCT00904826
Apr-13 February 8, 2010 May 13, 20Retinal Consultants o https://ClinicalTrials.gov/show/NCT01064505
Aug-24 November 13, 2020 April 3, 20 Sydney Brain Mind Cen https://ClinicalTrials.gov/show/NCT04626921
Aug-23 March 17, 2022 March 17, Peking Union Medicalhttps://ClinicalTrials.gov/show/NCT05284175
Jun-09 October 15, 2008 February 2Sanofi-Aventis Adminihttps://ClinicalTrials.gov/show/NCT00772525
NCT01721161
NCT04762017
NCT00856635
NCT01987167
NCT03630497
NCT01337986
NCT02657915
NCT02833142
NCT00179478
NCT00501748
NCT01777412
NCT04201262
NCT04155424
NCT02003144
NCT02073279
NCT02028884
NCT05145361
NCT04670770
NCT05730699
NCT05551598
NCT05549258
NCT05346354
NCT05314010
NCT05269667
NCT05199688
NCT04660539
NCT04629274
NCT04227470
NCT04146285
NCT05432713
NCT03330418
NCT02200770
NCT03138421
NCT01845584
NCT05840055
NCT05974293
NCT00304291
NCT02166346
NCT04561557
NCT01759602
NCT00904826
NCT01064505
NCT04626921
NCT05284175
NCT00772525
OCS 05 - Oculis
Corticotropin - Mallinckrodt
You might also like
- I. Introduction - Basic PharmacologyDocument4 pagesI. Introduction - Basic Pharmacologymdgayas70100% (2)
- Report ViewerDocument1 pageReport ViewerImran KhalidNo ratings yet
- MASIMODocument2 pagesMASIMOnihayah hanafiNo ratings yet
- Lampiran Surat Rapid Test Sampe Desember FIXDocument48 pagesLampiran Surat Rapid Test Sampe Desember FIXChristianHanjokarNo ratings yet
- Drug FactsDocument5 pagesDrug FactssaravananNo ratings yet
- 4 BahubaliDocument3 pages4 BahubaliDeepak VermaNo ratings yet
- FormatCARS KAAUH Alqahtani 106Document8 pagesFormatCARS KAAUH Alqahtani 106ZEYNONo ratings yet
- Prodia Blood Test Results - Heavy Metals and ParasitesDocument2 pagesProdia Blood Test Results - Heavy Metals and ParasitesMichael Sheehan AlahouzosNo ratings yet
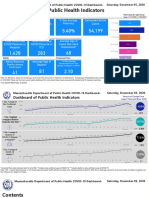
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- Dr. Nur Farhanah - SPEED TetanusDocument24 pagesDr. Nur Farhanah - SPEED TetanusJonathan IngramNo ratings yet
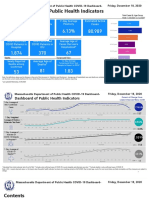
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- Master Rotation Plan-3Rd Year Basic-Bsc Nursing: Indira Gandhi College of NursingDocument5 pagesMaster Rotation Plan-3Rd Year Basic-Bsc Nursing: Indira Gandhi College of Nursingkuruvagadda sagarNo ratings yet
- SR.05. Epid Covid-19 PTVI Per 30 Sept 2020Document26 pagesSR.05. Epid Covid-19 PTVI Per 30 Sept 2020Rahmat SaputraNo ratings yet
- Pediatric Early Warning ScoreDocument2 pagesPediatric Early Warning ScoreSherif MleekNo ratings yet
- ItsOurBaby File-2Document5 pagesItsOurBaby File-2AA4884No ratings yet
- مناقشة ١اغسطس ٢٠٢١ PDFDocument299 pagesمناقشة ١اغسطس ٢٠٢١ PDFQq qNo ratings yet
- Adolescent TuberculosisDocument29 pagesAdolescent TuberculosistioNo ratings yet
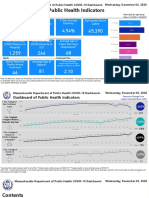
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- SwabAntigen 19052021 DB20210519063253512-1 UnlockedDocument1 pageSwabAntigen 19052021 DB20210519063253512-1 UnlockedAmien AfifNo ratings yet
- LF G3920SA 0010 Clinical ReportDocument16 pagesLF G3920SA 0010 Clinical ReportRoxana HermosoNo ratings yet
- SC - Wise Special Immu. (JE, TT) ReportDocument5 pagesSC - Wise Special Immu. (JE, TT) ReportAthish KumarNo ratings yet
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- 03-NS-2013 BangDanhGiaThoiGianThuViec Approved Version2.0Document1 page03-NS-2013 BangDanhGiaThoiGianThuViec Approved Version2.0hongchuyenNo ratings yet
- Serum: MR M Edukondalu Kphb9502Document2 pagesSerum: MR M Edukondalu Kphb9502Edukondalu MorlaNo ratings yet
- Course Lineup - College of MedicineDocument2 pagesCourse Lineup - College of MedicineRaymond TugayNo ratings yet
- Covidreportrtpcrtest YashDocument2 pagesCovidreportrtpcrtest YashYash ShahiNo ratings yet
- Pediatric Parameters and Equipment: Premie Newborn 6 Mo 1 Yr 2-3 Yr 4 - 6 Yr 7 - 10 Yr 11 - 15 Yr 16 YrDocument1 pagePediatric Parameters and Equipment: Premie Newborn 6 Mo 1 Yr 2-3 Yr 4 - 6 Yr 7 - 10 Yr 11 - 15 Yr 16 YrHòa ThảoNo ratings yet
- Alterações Sociais e Ocitocina No TEA, Higashida, 2019Document12 pagesAlterações Sociais e Ocitocina No TEA, Higashida, 2019Anuska ErikaNo ratings yet
- Coronavirus Dashboard 12/31/2020Document28 pagesCoronavirus Dashboard 12/31/2020KaitlynNo ratings yet
- Multiple Sclerosis HMRIDocument57 pagesMultiple Sclerosis HMRIDrNorNo ratings yet
- Cardiac EmergenciesDocument73 pagesCardiac EmergenciesKristine CaringalNo ratings yet
- OMMC Pedia Notes EDITEDDocument234 pagesOMMC Pedia Notes EDITEDEunice RiveraNo ratings yet
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJack PickellNo ratings yet
- Manaloto, Cid Marlon Libo 2211108539Document3 pagesManaloto, Cid Marlon Libo 2211108539Camille Marie ManalotoNo ratings yet
- The Validity of Two Versions of The GHQ in TheDocument7 pagesThe Validity of Two Versions of The GHQ in TheFarah SajidahNo ratings yet
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- Molecular Diagnostics: Report Status - Final Test Name Result Biological Ref. Interval UnitDocument2 pagesMolecular Diagnostics: Report Status - Final Test Name Result Biological Ref. Interval UnitAryan RathoreNo ratings yet
- EMS ResultDocument1 pageEMS ResultAparna VinodNo ratings yet
- Manuscript TBI 1Document8 pagesManuscript TBI 1Kevin TandartoNo ratings yet
- TREO Annual Clinical Update 2021 - PM-05517Document6 pagesTREO Annual Clinical Update 2021 - PM-05517Mircea Eugen MutuNo ratings yet
- Case SummaryDocument4 pagesCase Summarydivygupta198No ratings yet
- 3500720055_suman sahaDocument4 pages3500720055_suman saha55MESuman sahaNo ratings yet
- Ijhss-Format-An Exploratory Study On The Impact of Clusters On The Plastic Industry in Tamil NaduDocument6 pagesIjhss-Format-An Exploratory Study On The Impact of Clusters On The Plastic Industry in Tamil Naduiaset123No ratings yet
- ReportDeliverySamples LimitLISDocument9 pagesReportDeliverySamples LimitLISANTON SOKOLINo ratings yet
- Covid 19 Dashboard - 12 03 2020Document28 pagesCovid 19 Dashboard - 12 03 2020Rami Abou-SabeNo ratings yet
- U.S. Food & Drug Administration 10903 New Hampshire Avenue: Silver Spring, MD 20993Document42 pagesU.S. Food & Drug Administration 10903 New Hampshire Avenue: Silver Spring, MD 20993Mohammed SairawanNo ratings yet
- CRITILLNRIDEDocument2 pagesCRITILLNRIDEHarish ChandNo ratings yet
- In A Relationship and More!: Activity 4Document1 pageIn A Relationship and More!: Activity 4Justine Medellin100% (3)
- Test ReportsDocument2 pagesTest ReportsChandrasekhara Reddy TNo ratings yet
- Dashboard of Public Health IndicatorsDocument27 pagesDashboard of Public Health IndicatorsJohn WallerNo ratings yet
- Bicol MayDocument47 pagesBicol MayJoseph BaldonasaNo ratings yet
- Practicalskillschecklist 2017 - 2018 Week 13Document12 pagesPracticalskillschecklist 2017 - 2018 Week 13api-395143739No ratings yet
- Biophysical Properties in Glaucoma: Diagnostic TechnologiesFrom EverandBiophysical Properties in Glaucoma: Diagnostic TechnologiesIngrida JanulevicieneNo ratings yet
- Nuclear Medicine Technology: Review Questions for the Board ExaminationsFrom EverandNuclear Medicine Technology: Review Questions for the Board ExaminationsNo ratings yet
- Your Next Great Stock: How to Screen the Market for Tomorrow's Top PerformersFrom EverandYour Next Great Stock: How to Screen the Market for Tomorrow's Top PerformersRating: 3 out of 5 stars3/5 (1)
- CollaborationsDocument8 pagesCollaborationsjai.kumar.maingiNo ratings yet
- Bone Cancer - 1 - 2024Document2 pagesBone Cancer - 1 - 2024jai.kumar.maingiNo ratings yet
- Direct Medical Costs of Hospitalized Patients With.16Document3 pagesDirect Medical Costs of Hospitalized Patients With.16jai.kumar.maingiNo ratings yet
- List of Hospital - Pan India 28496Document660 pagesList of Hospital - Pan India 28496jai.kumar.maingiNo ratings yet
- Clinical Research Overview DownloadDocument13 pagesClinical Research Overview DownloadWILLIAM RICARDO CASTAÑEDA VARGASNo ratings yet
- 2010 WCBP CherneyBarryDocument27 pages2010 WCBP CherneyBarryAjay KumarNo ratings yet
- IB Cabotegravir v10Document285 pagesIB Cabotegravir v10Luana MarinsNo ratings yet
- Cowen ArenaDocument146 pagesCowen ArenaPhil MurrayNo ratings yet
- SaNOtize COVID Whitepaper Oct 2020Document2 pagesSaNOtize COVID Whitepaper Oct 2020Monte AltoNo ratings yet
- Early Development Best Practices For Stability-Regulatory PerspectiveDocument20 pagesEarly Development Best Practices For Stability-Regulatory PerspectiveMartin CelestinoNo ratings yet
- Clinical Trials Thesis TopicsDocument4 pagesClinical Trials Thesis Topicsshannonjoyarvada100% (2)
- Stability Guideline PDFDocument32 pagesStability Guideline PDFMostofa Rubal100% (1)
- 2019-01 Introduction To Pharmaceutical EngineeringDocument7 pages2019-01 Introduction To Pharmaceutical EngineeringAndy HermanNo ratings yet
- MDMA-IB-15th-Edition FINAL 13MAR2023 MRCDocument200 pagesMDMA-IB-15th-Edition FINAL 13MAR2023 MRCSi MarNo ratings yet
- Gingipain InhibitorsDocument13 pagesGingipain Inhibitorssbs99ifpNo ratings yet
- EAPP - Group 2 - Outline of How Drugs Are StudiedDocument1 pageEAPP - Group 2 - Outline of How Drugs Are Studiedsamantha B.No ratings yet
- Concise Notes in Oncology 2005Document186 pagesConcise Notes in Oncology 2005Mohammed FathyNo ratings yet
- DNA Breakthrough by SlidesgoDocument44 pagesDNA Breakthrough by SlidesgoAnnisa Nurul HasanahNo ratings yet
- Opening and Welcome SpeechDocument3 pagesOpening and Welcome SpeechRodnel MonceraNo ratings yet
- Ae / de / Es / Mba / MM / QM / Pom / Pdba ZC 523 Project Management - Ii Sem 2020 - 2021Document78 pagesAe / de / Es / Mba / MM / QM / Pom / Pdba ZC 523 Project Management - Ii Sem 2020 - 2021VINU V CHALAMNo ratings yet
- S. No. Division Name Purpose Name Fee Paid As Per GSR 1193Document9 pagesS. No. Division Name Purpose Name Fee Paid As Per GSR 1193VIJAY YADAVNo ratings yet
- Effectiveness of Individualized Homeopathic Treatment in Perennial Allergic Rhinitis (PAR)Document4 pagesEffectiveness of Individualized Homeopathic Treatment in Perennial Allergic Rhinitis (PAR)sai sindhujaNo ratings yet
- Ethics Guideline For Biomedical Research and Human Participant Schedule YDocument23 pagesEthics Guideline For Biomedical Research and Human Participant Schedule YNikita NehaNo ratings yet
- Pharmaceutical Business Management: Group 9Document25 pagesPharmaceutical Business Management: Group 9Dhanashree TeliNo ratings yet
- 6.toxicokinetics in Animal Toxicology Studies.01Document18 pages6.toxicokinetics in Animal Toxicology Studies.01Manu m rNo ratings yet
- Dosage Chapter 1 PDFDocument4 pagesDosage Chapter 1 PDFLena EmataNo ratings yet
- SOFIE FAP Webinar 2023Document42 pagesSOFIE FAP Webinar 2023Shibin JayakrishnanNo ratings yet
- Untitled DocumentDocument38 pagesUntitled Documentrobymuiruri42No ratings yet
- Drug Discovery and Development: Understanding The R&D ProcessDocument14 pagesDrug Discovery and Development: Understanding The R&D ProcessBandita DattaNo ratings yet
- Metastatic Colorectal Carcinoma After Second Progression and The Role of Trifluridine-Tipiracil (TAS-102) in SwitzerlandDocument8 pagesMetastatic Colorectal Carcinoma After Second Progression and The Role of Trifluridine-Tipiracil (TAS-102) in SwitzerlandAmina GoharyNo ratings yet
- Start Up Compendium 2023Document141 pagesStart Up Compendium 2023tau desiNo ratings yet
- Webinar Presentation 100909Document31 pagesWebinar Presentation 100909D'rulz KhenatNo ratings yet
- NMRR Data Elements For Submission Version 2Document12 pagesNMRR Data Elements For Submission Version 2Yeoh Jia LimNo ratings yet