Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
2 viewsWrite up_Olfctry epith(1).docx
Write up_Olfctry epith(1).docx
Uploaded by
peachybonyCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Evidence For Evolution WorksheetDocument2 pagesEvidence For Evolution Worksheetapi-29999681550% (4)
- ADOPT - UT - Miller Levine Biology - 2014Document10 pagesADOPT - UT - Miller Levine Biology - 2014Diana Macias0% (1)
- M.SC Food Tech Lecture NotesDocument115 pagesM.SC Food Tech Lecture NotesBabli SharmaNo ratings yet
- Introduction To Insect Sensory Organs As A Model System in Sensory Physiology and Developmental BiologyDocument3 pagesIntroduction To Insect Sensory Organs As A Model System in Sensory Physiology and Developmental BiologypajtgocjNo ratings yet
- Ls1 Final Study GuideDocument7 pagesLs1 Final Study GuideMinh NguyenNo ratings yet
- Phylum CtenophoraDocument23 pagesPhylum CtenophoramsnidanusratNo ratings yet
- 27123958Document13 pages27123958Al-ameen OlawunmiNo ratings yet
- Morphology of InsectDocument31 pagesMorphology of InsectSera Esra PaulNo ratings yet
- ZOO211 MATERIALS With IntroductionDocument32 pagesZOO211 MATERIALS With IntroductionAdwale oluwatobi festusNo ratings yet
- Cleavage Formation and Blastulation: Lesson 2.3: Animal DevelopmentDocument8 pagesCleavage Formation and Blastulation: Lesson 2.3: Animal DevelopmentWestminster AbbeyNo ratings yet
- Aurelia MaqniDocument6 pagesAurelia MaqniEhm MelsNo ratings yet
- University of The Punjab: BS-Chemistry 1st Semester-2021 Zoology-Invertebrates Diversity (ZOOL-101)Document6 pagesUniversity of The Punjab: BS-Chemistry 1st Semester-2021 Zoology-Invertebrates Diversity (ZOOL-101)basortusu13No ratings yet
- THESIS Paper Writing (Experiment)Document15 pagesTHESIS Paper Writing (Experiment)Ali Akand AsifNo ratings yet
- Gen Bio ReviewerDocument15 pagesGen Bio ReviewerAwaawa D orianoNo ratings yet
- PP13 Diversity of AnimalsDocument83 pagesPP13 Diversity of AnimalsmatodziNo ratings yet
- Annelida and EchinodermataDocument8 pagesAnnelida and EchinodermataMellya RizkiNo ratings yet
- To Study The External Features and Digestive System of CockroachesDocument28 pagesTo Study The External Features and Digestive System of CockroachesMinish KatkarNo ratings yet
- Classification Notes (Students)Document11 pagesClassification Notes (Students)Ye YulianNo ratings yet
- EntomologyDocument50 pagesEntomologyapi-19960301100% (1)
- Q2 L4 HandoutDocument4 pagesQ2 L4 HandoutmiatamayoqtNo ratings yet
- ZOO 211 - A ClassDocument11 pagesZOO 211 - A Classmusanafisat593No ratings yet
- Introductory Chapter: The Complex World of AntsDocument6 pagesIntroductory Chapter: The Complex World of AntsRustashNo ratings yet
- (Jumat) WO & LO SSS Week 5 Felicia Lidya 01071180081Document13 pages(Jumat) WO & LO SSS Week 5 Felicia Lidya 01071180081lala landNo ratings yet
- Segers 2003 BDocument16 pagesSegers 2003 BEstefania SarangoNo ratings yet
- Olfactory PathwayDocument6 pagesOlfactory PathwayJean Pierre Chastre LuzaNo ratings yet
- CRINOIDEADocument12 pagesCRINOIDEAAlfina Wulandari100% (2)
- Phylum Annelida 4Document22 pagesPhylum Annelida 4Ahmed OrabyNo ratings yet
- Study QuestionDocument24 pagesStudy QuestionEbookslatinosEbookslatinosNo ratings yet
- Biology Topic: 1.1 Study Living ThingsDocument5 pagesBiology Topic: 1.1 Study Living Thingsazizul hasanNo ratings yet
- Carbon FixationDocument6 pagesCarbon FixationMridul Kumar BarmanNo ratings yet
- Hemichordates: Is Divided Into 3 PartsDocument8 pagesHemichordates: Is Divided Into 3 Partstaimoor shahzadNo ratings yet
- Zoology BLMDocument27 pagesZoology BLMsanjeevtsms2023No ratings yet
- Nematode StructureDocument31 pagesNematode StructureShadab HanafiNo ratings yet
- Rearing of Insect ReviewerDocument14 pagesRearing of Insect ReviewerrjamiirsuruelosNo ratings yet
- The Phylum Annelida: A Short IntroductionDocument3 pagesThe Phylum Annelida: A Short IntroductionTI Journals PublishingNo ratings yet
- Lec2 BsbioDocument7 pagesLec2 BsbioKathleen SaldonNo ratings yet
- Phylum AnimaliaDocument28 pagesPhylum Animaliaapi-310714826No ratings yet
- Bacteria, Domain Archaea, and Domain Eukarya. Each Domain Can BeDocument6 pagesBacteria, Domain Archaea, and Domain Eukarya. Each Domain Can BeJonathan Mohler-FariaNo ratings yet
- Semester II Botany Major Class Notes /hints On Lichens: Their Meaning, Characteristics, Types, Classfication EtcDocument17 pagesSemester II Botany Major Class Notes /hints On Lichens: Their Meaning, Characteristics, Types, Classfication EtcJán ŠormanNo ratings yet
- UntitledDocument4 pagesUntitledAbby Joy SeniningNo ratings yet
- Exercise7 (Histo) Digestive System EditedDocument8 pagesExercise7 (Histo) Digestive System EditedChristi Lorraine LayogNo ratings yet
- CH-01 FinalDocument16 pagesCH-01 FinalRaJa100% (1)
- Other Trending Diciplines: Group 6Document15 pagesOther Trending Diciplines: Group 6Jerome MilladoNo ratings yet
- Phylum PoriferaDocument18 pagesPhylum Poriferagonoles81No ratings yet
- Respiratory SystemDocument15 pagesRespiratory SystemJovelou MihangosNo ratings yet
- Allama Iqbal Open University IslamabadDocument16 pagesAllama Iqbal Open University IslamabadAbdullah NagraNo ratings yet
- Crissman Epithelial ObjectivesDocument6 pagesCrissman Epithelial Objectiveskkonci01No ratings yet
- Non Mammalian BarbelsDocument41 pagesNon Mammalian BarbelsDamian HaydenNo ratings yet
- 16tasteandsmell TRDocument56 pages16tasteandsmell TRBharat NarumanchiNo ratings yet
- Bio System SummariesDocument52 pagesBio System Summariesxara zinkNo ratings yet
- Body System II 5 Respiratory 1Document27 pagesBody System II 5 Respiratory 1rrq8cwk2gnNo ratings yet
- Biology AQA Chapter 1 Questions and AnswersDocument18 pagesBiology AQA Chapter 1 Questions and Answersmahamed100% (1)
- Science FinalDocument96 pagesScience FinalChitransh GuptaNo ratings yet
- CHAPTER 6 Animalia (Invertebrate)Document17 pagesCHAPTER 6 Animalia (Invertebrate)mottong100% (1)
- Characteristics of Phylum Porifera (Sponges)Document15 pagesCharacteristics of Phylum Porifera (Sponges)Pralex PrajapatiNo ratings yet
- علم الحشراتDocument82 pagesعلم الحشراتshereefadoma96No ratings yet
- Lecture 16 - Annelids & Nematoda: Phylum Annelida Key ConceptDocument19 pagesLecture 16 - Annelids & Nematoda: Phylum Annelida Key ConceptmegsofmemoryNo ratings yet
- COL3Document11 pagesCOL3Clefford CorporalNo ratings yet
- Organisation of LifeDocument46 pagesOrganisation of LifesanthaNo ratings yet
- Zoology BLMDocument28 pagesZoology BLMzeeshanzaka489No ratings yet
- A Guide for the Dissection of the Dogfish (Squalus Acanthias)From EverandA Guide for the Dissection of the Dogfish (Squalus Acanthias)No ratings yet
- Potency 11.7.18-1Document2 pagesPotency 11.7.18-1peachybonyNo ratings yet
- Development of XenopusDocument25 pagesDevelopment of XenopuspeachybonyNo ratings yet
- P43201381732602062023091312Document1 pageP43201381732602062023091312peachybonyNo ratings yet
- AltruismDocument3 pagesAltruismpeachybonyNo ratings yet
- Scan 24 Jul 23 10 15 38Document2 pagesScan 24 Jul 23 10 15 38peachybonyNo ratings yet
- 2018 Sem 1 QuestionsDocument10 pages2018 Sem 1 QuestionspeachybonyNo ratings yet
- DR Linda Molecular BiosDocument73 pagesDR Linda Molecular BiosValency BathoNo ratings yet
- Biotechnology8 q3 Mod2 Manipulation-of-Genetic-MaterialDocument16 pagesBiotechnology8 q3 Mod2 Manipulation-of-Genetic-Materialunc b50% (6)
- Biotechnology Principles and Processes - NotesDocument8 pagesBiotechnology Principles and Processes - NotesSquad 4 GamingNo ratings yet
- CyberbiosecurityDocument3 pagesCyberbiosecuritywatson191No ratings yet
- Bacterial Colony Isolation Using Serial Dilution TechniquesDocument2 pagesBacterial Colony Isolation Using Serial Dilution TechniquesAl Jay Mejos80% (5)
- Chang 2014 Social Isolation Induced Increase in NMDA Receptors in The Hippocampus Exacerbates Emotional Dysregulation in MiceDocument12 pagesChang 2014 Social Isolation Induced Increase in NMDA Receptors in The Hippocampus Exacerbates Emotional Dysregulation in MiceMarilyn GarciaNo ratings yet
- Effects of Chromolaena Odorata Leaf Meal SupplemenDocument10 pagesEffects of Chromolaena Odorata Leaf Meal SupplemenEmmanuel OwonaNo ratings yet
- Kharghar, Navi Mumbai - 410 210Document1 pageKharghar, Navi Mumbai - 410 210Ankit TiwariNo ratings yet
- Grammar 1 MinDocument8 pagesGrammar 1 Minlila bNo ratings yet
- Botany 1Document11 pagesBotany 1Richard NepomucenoNo ratings yet
- Hodges - The Guild Handbook of Scientific IllustrationDocument612 pagesHodges - The Guild Handbook of Scientific IllustrationGermán Huici Escribano100% (1)
- Nascimento Et Al. 2017 A Biologit S Guide To Bayesian Phylogenetic AnalysisDocument21 pagesNascimento Et Al. 2017 A Biologit S Guide To Bayesian Phylogenetic Analysisguilherme.rodrigues.bioNo ratings yet
- Practical Manual BIO 1141: University of Venda Department of Biological SciencesDocument42 pagesPractical Manual BIO 1141: University of Venda Department of Biological SciencesBelfry MantshwaneNo ratings yet
- قلق الموت من السرطانDocument212 pagesقلق الموت من السرطانBouchareb AhmedNo ratings yet
- B2 Reading 4Document4 pagesB2 Reading 4Herminio Garcia-RiañoNo ratings yet
- MicrobiologyDocument2 pagesMicrobiologyIrfan SalimNo ratings yet
- Synthesis and Characterization of Silica Microcapsules With Active Compounds 2% Chlorhexidine Using Sodium Alginate and Chitosan Coating As Medicament of Root Canal InfectionDocument7 pagesSynthesis and Characterization of Silica Microcapsules With Active Compounds 2% Chlorhexidine Using Sodium Alginate and Chitosan Coating As Medicament of Root Canal InfectionSEP-PublisherNo ratings yet
- Reincarnated As A Dragon Hatchling Volume 3Document290 pagesReincarnated As A Dragon Hatchling Volume 3Phil100% (1)
- 10-20 CAPTODAY HematologyAnalyzersDocument12 pages10-20 CAPTODAY HematologyAnalyzersalexandre larmagnacNo ratings yet
- Unit 2 Octopus VocabularyDocument6 pagesUnit 2 Octopus VocabularyBadass PolapainNo ratings yet
- 609 1121 1 SMDocument9 pages609 1121 1 SMpengembangan disbudparkabkediriNo ratings yet
- Anaphy Free Sample ExamDocument5 pagesAnaphy Free Sample ExamNurse UtopiaNo ratings yet
- 1erTareaMicroscopíasLópez Marmolejo Clere MishellDocument4 pages1erTareaMicroscopíasLópez Marmolejo Clere Mishellclere02marmolejoNo ratings yet
- Class 7 Science Sample Paper Term 2 Set 1 2021 2022Document2 pagesClass 7 Science Sample Paper Term 2 Set 1 2021 2022akashkushhNo ratings yet
- F3 End Term 2 Exams PDFDocument253 pagesF3 End Term 2 Exams PDFPAMAJA100% (1)
- Bioprospecting of Microbial Diversity Challenges and Applications in Biochemical Industry Agriculture and Environment Protection Pradeep Verma Maulin P Shah Full ChapterDocument52 pagesBioprospecting of Microbial Diversity Challenges and Applications in Biochemical Industry Agriculture and Environment Protection Pradeep Verma Maulin P Shah Full Chapterkristan.wade654100% (5)
- 2210030313FC Apipah ApriantiDocument1 page2210030313FC Apipah Apriantitomo bukakaNo ratings yet
- DescargaDocument22 pagesDescargaKemnet Aguirre ValenzuelaNo ratings yet
Write up_Olfctry epith(1).docx
Write up_Olfctry epith(1).docx
Uploaded by
peachybony0 ratings0% found this document useful (0 votes)
2 views6 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
2 views6 pagesWrite up_Olfctry epith(1).docx
Write up_Olfctry epith(1).docx
Uploaded by
peachybonyCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 6
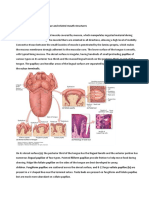
Structure of Olfactory organ in fishes
The olfactory organ is a peripheral part of the olfactory system.
It is a paired structure.
The olfactory organ in most fish species is well developed and is located on the dorsal surface of the
head to the rostrum and medial of the eyes.
Water gets into the olfactory organ, the nasal or olfactory cavity via the anterior opening or the anterior
nostril, and gets out via the posterior olfactory opening or posterior nostril.
These openings are divided by a skin nasal bridge or nasal bridge. The nasal bridge of many fishes has a
skinny nasal ridge directing water into the anterior nostril.
Olfactory lamellae forming the olfactory rosette are located at the bottom of the nasal cavity. The
surface of the lamella is covered by the olfactory epithelium. The basic function of olfactory lamellae is
increase the area of the olfactory epithelium bearing the receptor cells which perceive chemical stimuli.
There exist several types of olfactory rosettes differing by the position of lamellae: radial (Acipenseridae,
Esocidae), arrow-like (Cyprinidae, Salmonidae), parallel (Pleuronectidae, Lepisosteus, Lophiiformes), and
bilateral (Anguilliformes, Siluriformes, Dipnoi, Amia, Chondrychthyes). There exist numerous transitory
forms between these basic types (Fig 12). Closely related species of fish, for example, belonging to the
same family, usually have olfactory rosettes of the same or similar type (Chen and Arratia, 1994).
This general scheme of the olfactory organ may significantly differ in representatives of different
taxonomic groups of fish (Zeiske et al., 1992).
Cellular composition of olfactory organ in fish
Cells of different types are included into the epithelium, mostly receptor, support, mucous, and basal.
Receptor cells. Olfactory receptor cells are the primary sensitive cells, that is, they represent nervous
cells (sensory neurons). Receptor cells have a thick dendrite, which goes out from the central (nuclear)
part of the neuron and reaches the surface of the epithelium. The apical part of the dendrite terminates
at a rounded tip (knob) which projects over the surface of the epithelium. In fish, receptor cells in the
olfactory epithelium are represented by two different types: ciliated and microvillous receptor cells
(Bernshtein, 1977; Yamamoto, 1982; Zeiske et al., 1992). Recently, a third type of cells has been found in
the olfactory epithelium of different fish species: crypt cells. They are relatively rare and their knob does
not reach the surface of the epithelium but opens in a small cavity located directly under the external
surface of it (Hansen and Zeiske, 1998; Hansen and Finger, 2000; Zeiske et al., 2003). The knob of
receptor ciliated cells bears weakly motile cilia. Their number ranges from 4 to 20 and the length reaches
5–10 µm, and diameter, 0.25–0.30 µm. The number of microvilli in microvillous receptor cells is
significantly larger, from 30 to 80. Their length ranges from 0.5 to 3–5 µm, diameter about 0.1 µm
(Bannister, 1965; Gemne and Døving, 1969; Schulte, 1972; Bronshtein, 1977; Sorensen and Caprio,
1998). Numerous ciliated–microvillous receptor cells can be found in the olfactory epithelium of certain
fishes (e.g. in sturgeons), the knobs of these cells bear both cilia and microvilli (Pyatkina, 1975, 1976).
Cilia of receptor cells move slowly and asynchronously and are usually stretched along the surface of the
epithelium. A long central process, the axon, goes out from the opposite side of the receptor cell (Fig.
14). Special protein receptor molecules are contained in the cellular membrane of cilia and microvilli.
When the receptor protein contacts the olfactory substance, a complicated cascade of molecular
transformations occurs in the cell, which is called the signal transduction process. This process changes
the functioning of the ion channels in the cell, generation of receptor potential, and its distribution as an
electric nervous impulse from the generation location to the primary and secondary olfactory centers
(Brand and Bruch, 1992). The central (nuclear) part of the receptor cell is located in the olfactory
epithelium at different levels. Five such layers are usually distinguished in the epithelium. The lower the
layer with the nucleus of the receptor cell, the longer its dendrite, which reaches the outer surface of the
epithelium. It was found that the nuclear part of ciliated receptor cells is usually located in deeper layers
of the olfactory epithelium than in microvillous. Therefore, its dendrites are longer. The shortest
dendrites are characteristic of crypt receptor cells, which are located in the upper layer of the epithelium
(Hansen and Finger, 2000; Hamdani et al., 2001a; Hamdani and Døving, 2002). Receptor cells occur in
the olfactory epithelium in areas covering the side surface of olfactory lamellae. They are absent on
ridges of lamellae, and on vaults of the olfactory cavity. Areas of the olfactory epithelium bearing
receptor cells are named receptor zones or receptor (sensory) epithelium. These zones alternate with
zones of non-receptor epithelium. The distribution of receptor cells at the lateral surface of lamellae in
different fish species may differ. Continuous, large-zone, fine-zone, and irregular types of sensory
epithelium distribution are usually distinguished (Fig. 15) (Yamamoto and Ueda, 1979; Yamamoto, 1982).
The average density of receptor cells in the receptor epithelium ranges from 20–30 thousand per mm2
to 400–500 thousand per mm2 , and their total number ranges between several dozens to a hundred
thousand to several dozen million or more (Holl, 1965; Holl et al., 1970; Zeiske et al., 1976, 1979;
Thommesen, 1983; Devitsyna and Attar, 1988). The density of ciliated and microvillous cells may differ in
different areas of olfactory lamellae but the total number of ciliated cells is two times more than
microvillous (Thommesen, 1983). The largest number of receptor cells has been found in the common
wels Silurus glanis which approach certain mammals (dog) with well-developed olfaction (Table 3). It
should be noted that linear growth in fish and the associated increase of the number of receptor cells
occurs throughout their whole life, therefore, large individuals have larger olfactory organs and more
receptor cells (Pashchenko, 1986). All types of receptor cells are present in most groups of fish, teleosts,
cartilaginous, Chondrostei (sturgeons), and in myxini (Theissen, 1976; Yamamoto, 1982; Eistein, 1982;
Zeiske et al., 1992). But Elasmobranchs and some dip receptor cells, whereas Polypteridae, coelacanths,
Crosspterigi, the river lamprey Lampetra fluviatilis, marine lamprey Pteromyzon marinus and probably
other lampreys have only ciliated cells (Thornhill, 1967; Reese and Brightman, 1970; Theissen, 1972;
Bronstein, 1976; Zeiske et al., 1987; Van Denbossche et al., 1995). Ciliated, microvillous and crypt cells
specialize in perception of different groups of chemical stimuli (Thommesen, 1982, 1983; Hamdani et al.,
2000, 2001a, 2001b; Sato and Suzuki, 2001; Hamdani and Døving, 2002, 2003; Weltzen et al., 2003).
Within all these groups, the cells probably have further functional specialization. Unlike amphibians,
reptiles and other vertebrates, this specialization in fish is not associated with zonality in the distribution
of different receptor types in the olfactory epithelium (Yamamoto, 1982; Ngai et al., 1993).
Supporting cells. Supporting cells surrounding the receptor cells are represented in the olfactory
epithelium by two different types: the supporting cells, i.e., cells with a free surface not bearing any
specialized structures, and ciliated cells, i.e., cells bearing numerous kinocilia on the surface facing the
cavity of the nasal sac. The number of kinocilia in one ciliate cell may reach several dozen, they have a
length of up to 5 µm and a diameter 0.2–0.3 µm. Kinocilia of the ciliated cells move synchronously, and
their activity is responsible for the ventilation of the olfactory cavity in areas located between close
lamellae of the olfactory rosette, and in many fish species, ventilation of the whole olfactory organ
(Døving, 1986). The function of these cells is very important because they provide for the transport of
water which carries the signal to the receptor cells.
Goblet cells. Goblet cells are very numerous in the olfactory epithelium. They are located in its upper
layer and may differ in size. Small and large mucous cells are usually distinguished. The function of these
cells is associated with the production of a special olfactory mucus, which covers the surface of the
olfactory epithelium. This mucous layer or glycocalix completely covers cilia and microvilli of the receptor
cells and the bases of kinocilia of the ciliated cells. The olfactory mucus includes mucopolysaccharides,
proteins, lipids, and different ions (Doroshenko and Pinchuk, 1974; Bronshtein, 1977). It is hypothesized
that glycocalix plays an important role in chemoreception and that molecules of signal substances are
first introduced into the mucus, interact with its components, and only then reach the receptor areas in
the cellular membrane of cilia and microvilli. The mucus creates optimal conditions for the molecular
processes involved into the interaction between the sig nal substance (ligand) and the receptor proteins
(Sorensen and Caprio, 1998). It is thought that the olfactory mucus has important protective functions,
protecting flagella and microvilli of receptor cells from mechanical disturbances by small particles
transmitted by water currents. The important protective function of the mucous is revealed by the fact
that secretion of olfactory mucus significantly increases when the water is contaminated by various
chemical agents (detergents, heavy metals). The production of olfactory mucus in fish of the same family
is most pronounced in marine (cod Gadus morhua, navaga Eleginus navaga) than in freshwater (burbot
Lota lota) representatives (Devitsyna, 1972). Basal cells. Basal cells are located in the deepest layer of the
olfactory epithelium at the basal membrane. New cells of the olfactory epithelium, receptor, supporting,
and mucous, having limited longevity, develop and differentiate from basal cells. Receptor cells are
renewed every 7–10 days. Old cells of the epithelium degenerate and their content is either secreted
into the nasal cavity or is phagocytized by lymphatic cells (Graziadei and Monti-Graziadei, 1978;
Cancalon, 1982; Pashchenko and Kasumyan, 1984). The ability of the olfactory epithelium, primarily the
receptor cells, for constant renewal is an extremely important adaptation, allowing the fish to retain the
capacity to obtain olfactory information when the probability of receptor cell destruction and
disturbances is very high. The olfactory epithelium, including the receptor cells, directly opens into the
environment and is weakly protected from its negative effects. Particles suspended in water may damage
the epithelium (abrasive effects). Iridioviruses and bacteria, as well as different parasitic organisms,
microsporidia, fungi, and small crustaceans may also cause significant disturbances of the olfactory
epithelium. Receptor cells may be disturbed after a sharp change of water salinity in anadromous and
catadromous migrants. Deep structural disturbances and complete degeneration of many elements of
the olfactory epithelium may be caused by chemical contaminants (detergents, heavy metals, low pH)
(Bertmar, 1972; Devitsyna, 1977; Doroshenko and Pinchuk, 1978; Pashchenko and Kasumyan, 1984;
Baatrup and Døving, 1990; Klaprat et al., 1992; Morrison and Plumb, 1994; Watson et al., 1998).
Neuronal connection of olfactory organ in fishes
The Olfactory Nerve and the Olfactory Tract.
Axons of all receptor cells after the basal membrane of the olfactory epithelium are joined into the single
nerve, the n. olfactorius (pair I of brain nerves). The olfactory nerve links the olfactory organ with the
primary olfactory centers: paired olfactory bulbs (bulbus olfactorius) to which the olfactory information
comes as electric impulses (Fig. 16). Nervous fibers, i.e., axons of receptor cells, branch in the olfactory
bulb and form a surface fibrous layer. The glomerular layer of the olfactory bulb, formed by glomerules,
is located under it. Glomerules represent a complex interlacement of myriad branches of the olfactory
nerve and dendrites of mitral cells interacting with synaptic contacts (Døving, 1986; Satou, 1992;
Sorensen and Caprio, 1998). The relationships between the number of axons of receptor cells and the
number of mitral cells ranges from 40 : 1 in verkhovka Leucaspius delineatus to 1000/4000 : 1 in cod
Gadus morhua, blue bream Abramis ballerus, and bream Abramis brama. In the common wels Silurus
glanis, the relationship between these cells exceeds 12000 : 1 (Døving and Gemne, 1965; Devitsyna,
1977; Devitsyna and Attar, 1988). It is thought that the higher the ratio, the more developed are the
functional capacities of the olfactory system (Døving, 1986). The layer of mitral and ruffed cells is located
below the layer of glomerulas. Each intercalary cell connects several mitral cells and each mitral cell, in
turn, may have contacts with different intercalary cells, which causes the formation of large neuronal
assemblages. An aggregation of granular cells forming the anterior olfactory nucleus (nucleus olfactorius
anterior) is situated under the layer of mitral and intercalary cells. Granular cells have synaptic contacts
with mitral and intercalary cells. Centrifugal effects from the secondary olfactory center (nucleus
olfactorius posterior), located in the telencephalon and involved in the central regulation of the olfactory
bulb, affect granular cells. The primary and secondary olfactory centers are connected by a tract formed
by axons of mitral and intercalary cells, along with centrifugal fibers incoming to granular cells (they
belong to the system of the 0 pair of brain nerves, terminal nerve n. terminalis) (Sorensen and Caprio,
1998). Lateral and medial fibers are distinguished in the olfactory tract, each of which, in turn, is
separated into smaller segments (Sheldon, 1912; Holmgren, 1920; Døving and Gemne, 1965). Olfactory
bulbs are connected by interbulbar links passing via the forebrain (Satou, 1992). In most fish species,
olfactory bulbs are located adjacent to the forebrain and the olfactory nerve is quite long. Such bulbs are
called sessille. But in cyprinids, catfishes, cods, and sharks, holocephali and Mormyridae, olfactory bulbs
are remote from the forebrain and are adjacent to the olfactory organ (Døving, 1986). Olfactory nerves
in these fish are short but the olfactory tracts, going from olfactory bulbs to olfactory centers in the
forebrain, are of significant length. Such bulbs are called stalked (Fig. 17). In Characidae and some other
fish, olfactory bulbs are located in the middle between the olfactory organ and the forebrain.
Morphological and functional zonality has been found in the olfactory bulb of fish in recent years (Hara
and Zang, 1996, 1998; Laberge and Hara, 2001; Nikonov and Caprio, 2001). For example, the axons of
flagellated receptor cells come to the ventral surface of the olfactory bulb, whereas axons of microvillous
receptor cells arrive at the dorsal surface (Morita and Finger, 1998). When the olfactory epithelium is
stimulated by different chemical substances, the electric activity is recorded in different areas of the
olfactory bulb. For example, in the channel catfish Ictalurus punctatus, electric activity goes to the
medial part of the olfactory bulb via axons of ciliated cells sensitive to fatty acids, and via axons of cells
sensitive to free amino acids, electric activity goes to the ventral part of the olfactory bulb (Hansen et al.,
2003). Signals causing different behavioral patterns come to secondary centers via different fibers of the
olfactory tract (Døving and Selset, 1980). For example, olfactory signals bringing about food search
behavior in the silver carp Carassius carassius come via the lateral fiber of the olfactory tract whereas
signals causing defensive behavior, via the medial segment of the medial fiber of the olfactory tract. The
lateral segment of the medial fiber may be involved in the mediation of chemical signals responsible for
the stimulation of sexual behavior (Døving and Selset, 1980; Stacey and Kyle, 1983; Hamdani et al., 2000,
2001b; Hamdani and Døving, 2002, 2003; Weltzein et al., 2003). Studies of topographic and functional
specialization in the olfactory system of fish are continued quite intensely. They are very important for
understanding the evolutionary pathways of the secondary olfactory system: the vomeronasal (Jacobian)
organ, lacking in fish (Eistein, 1992). In mammals, this highly specialized system is involved in
Functions of olfactory organ in fishes
Olfaction in fishes plays vital role in fishes in perceiving changes in their surrounding
environment. It facilitates resource procuring as it is closely related to feeding and reproductive
behavior, predator avoidance, homing migration, territory maintenance, and inter and intra
specific signal communication by the identification of alarm substances. Such functions are
dependent on stimulation of olfactory receptor neurons (ORNs), which are highly sensitive to
changes of environmental factors (Hara, 1986). Following the diversity of habitats and ecological
niches that fishes occupy, the olfactory organs of fishes exhibit differential morphology,
structural organization, and distribution of the epithelium, and cytological characteristics (Atta,
2013).
In fishes, Nasal-associated lymphoid tissue (NALT) serves as a mucosal inductive site for
humoral immune responses against antigen stimulation in mammals, is present also in teleosts.
IgT in teleosts is responsible for similar functions to those carried out by IgA in mammals.
Moreover, teleost NALT is known to contain B-cells and teleost nasal mucus contains
immunoglobulins (Igs). Yet, whether nasal B cells and Igs respond to infection remains
unknown. We hypothesized that water-borne parasites can invade the nasal cavity of fish and
elicit local specific immune responses (Yu et al. 2018).
You might also like
- Evidence For Evolution WorksheetDocument2 pagesEvidence For Evolution Worksheetapi-29999681550% (4)
- ADOPT - UT - Miller Levine Biology - 2014Document10 pagesADOPT - UT - Miller Levine Biology - 2014Diana Macias0% (1)
- M.SC Food Tech Lecture NotesDocument115 pagesM.SC Food Tech Lecture NotesBabli SharmaNo ratings yet
- Introduction To Insect Sensory Organs As A Model System in Sensory Physiology and Developmental BiologyDocument3 pagesIntroduction To Insect Sensory Organs As A Model System in Sensory Physiology and Developmental BiologypajtgocjNo ratings yet
- Ls1 Final Study GuideDocument7 pagesLs1 Final Study GuideMinh NguyenNo ratings yet
- Phylum CtenophoraDocument23 pagesPhylum CtenophoramsnidanusratNo ratings yet
- 27123958Document13 pages27123958Al-ameen OlawunmiNo ratings yet
- Morphology of InsectDocument31 pagesMorphology of InsectSera Esra PaulNo ratings yet
- ZOO211 MATERIALS With IntroductionDocument32 pagesZOO211 MATERIALS With IntroductionAdwale oluwatobi festusNo ratings yet
- Cleavage Formation and Blastulation: Lesson 2.3: Animal DevelopmentDocument8 pagesCleavage Formation and Blastulation: Lesson 2.3: Animal DevelopmentWestminster AbbeyNo ratings yet
- Aurelia MaqniDocument6 pagesAurelia MaqniEhm MelsNo ratings yet
- University of The Punjab: BS-Chemistry 1st Semester-2021 Zoology-Invertebrates Diversity (ZOOL-101)Document6 pagesUniversity of The Punjab: BS-Chemistry 1st Semester-2021 Zoology-Invertebrates Diversity (ZOOL-101)basortusu13No ratings yet
- THESIS Paper Writing (Experiment)Document15 pagesTHESIS Paper Writing (Experiment)Ali Akand AsifNo ratings yet
- Gen Bio ReviewerDocument15 pagesGen Bio ReviewerAwaawa D orianoNo ratings yet
- PP13 Diversity of AnimalsDocument83 pagesPP13 Diversity of AnimalsmatodziNo ratings yet
- Annelida and EchinodermataDocument8 pagesAnnelida and EchinodermataMellya RizkiNo ratings yet
- To Study The External Features and Digestive System of CockroachesDocument28 pagesTo Study The External Features and Digestive System of CockroachesMinish KatkarNo ratings yet
- Classification Notes (Students)Document11 pagesClassification Notes (Students)Ye YulianNo ratings yet
- EntomologyDocument50 pagesEntomologyapi-19960301100% (1)
- Q2 L4 HandoutDocument4 pagesQ2 L4 HandoutmiatamayoqtNo ratings yet
- ZOO 211 - A ClassDocument11 pagesZOO 211 - A Classmusanafisat593No ratings yet
- Introductory Chapter: The Complex World of AntsDocument6 pagesIntroductory Chapter: The Complex World of AntsRustashNo ratings yet
- (Jumat) WO & LO SSS Week 5 Felicia Lidya 01071180081Document13 pages(Jumat) WO & LO SSS Week 5 Felicia Lidya 01071180081lala landNo ratings yet
- Segers 2003 BDocument16 pagesSegers 2003 BEstefania SarangoNo ratings yet
- Olfactory PathwayDocument6 pagesOlfactory PathwayJean Pierre Chastre LuzaNo ratings yet
- CRINOIDEADocument12 pagesCRINOIDEAAlfina Wulandari100% (2)
- Phylum Annelida 4Document22 pagesPhylum Annelida 4Ahmed OrabyNo ratings yet
- Study QuestionDocument24 pagesStudy QuestionEbookslatinosEbookslatinosNo ratings yet
- Biology Topic: 1.1 Study Living ThingsDocument5 pagesBiology Topic: 1.1 Study Living Thingsazizul hasanNo ratings yet
- Carbon FixationDocument6 pagesCarbon FixationMridul Kumar BarmanNo ratings yet
- Hemichordates: Is Divided Into 3 PartsDocument8 pagesHemichordates: Is Divided Into 3 Partstaimoor shahzadNo ratings yet
- Zoology BLMDocument27 pagesZoology BLMsanjeevtsms2023No ratings yet
- Nematode StructureDocument31 pagesNematode StructureShadab HanafiNo ratings yet
- Rearing of Insect ReviewerDocument14 pagesRearing of Insect ReviewerrjamiirsuruelosNo ratings yet
- The Phylum Annelida: A Short IntroductionDocument3 pagesThe Phylum Annelida: A Short IntroductionTI Journals PublishingNo ratings yet
- Lec2 BsbioDocument7 pagesLec2 BsbioKathleen SaldonNo ratings yet
- Phylum AnimaliaDocument28 pagesPhylum Animaliaapi-310714826No ratings yet
- Bacteria, Domain Archaea, and Domain Eukarya. Each Domain Can BeDocument6 pagesBacteria, Domain Archaea, and Domain Eukarya. Each Domain Can BeJonathan Mohler-FariaNo ratings yet
- Semester II Botany Major Class Notes /hints On Lichens: Their Meaning, Characteristics, Types, Classfication EtcDocument17 pagesSemester II Botany Major Class Notes /hints On Lichens: Their Meaning, Characteristics, Types, Classfication EtcJán ŠormanNo ratings yet
- UntitledDocument4 pagesUntitledAbby Joy SeniningNo ratings yet
- Exercise7 (Histo) Digestive System EditedDocument8 pagesExercise7 (Histo) Digestive System EditedChristi Lorraine LayogNo ratings yet
- CH-01 FinalDocument16 pagesCH-01 FinalRaJa100% (1)
- Other Trending Diciplines: Group 6Document15 pagesOther Trending Diciplines: Group 6Jerome MilladoNo ratings yet
- Phylum PoriferaDocument18 pagesPhylum Poriferagonoles81No ratings yet
- Respiratory SystemDocument15 pagesRespiratory SystemJovelou MihangosNo ratings yet
- Allama Iqbal Open University IslamabadDocument16 pagesAllama Iqbal Open University IslamabadAbdullah NagraNo ratings yet
- Crissman Epithelial ObjectivesDocument6 pagesCrissman Epithelial Objectiveskkonci01No ratings yet
- Non Mammalian BarbelsDocument41 pagesNon Mammalian BarbelsDamian HaydenNo ratings yet
- 16tasteandsmell TRDocument56 pages16tasteandsmell TRBharat NarumanchiNo ratings yet
- Bio System SummariesDocument52 pagesBio System Summariesxara zinkNo ratings yet
- Body System II 5 Respiratory 1Document27 pagesBody System II 5 Respiratory 1rrq8cwk2gnNo ratings yet
- Biology AQA Chapter 1 Questions and AnswersDocument18 pagesBiology AQA Chapter 1 Questions and Answersmahamed100% (1)
- Science FinalDocument96 pagesScience FinalChitransh GuptaNo ratings yet
- CHAPTER 6 Animalia (Invertebrate)Document17 pagesCHAPTER 6 Animalia (Invertebrate)mottong100% (1)
- Characteristics of Phylum Porifera (Sponges)Document15 pagesCharacteristics of Phylum Porifera (Sponges)Pralex PrajapatiNo ratings yet
- علم الحشراتDocument82 pagesعلم الحشراتshereefadoma96No ratings yet
- Lecture 16 - Annelids & Nematoda: Phylum Annelida Key ConceptDocument19 pagesLecture 16 - Annelids & Nematoda: Phylum Annelida Key ConceptmegsofmemoryNo ratings yet
- COL3Document11 pagesCOL3Clefford CorporalNo ratings yet
- Organisation of LifeDocument46 pagesOrganisation of LifesanthaNo ratings yet
- Zoology BLMDocument28 pagesZoology BLMzeeshanzaka489No ratings yet
- A Guide for the Dissection of the Dogfish (Squalus Acanthias)From EverandA Guide for the Dissection of the Dogfish (Squalus Acanthias)No ratings yet
- Potency 11.7.18-1Document2 pagesPotency 11.7.18-1peachybonyNo ratings yet
- Development of XenopusDocument25 pagesDevelopment of XenopuspeachybonyNo ratings yet
- P43201381732602062023091312Document1 pageP43201381732602062023091312peachybonyNo ratings yet
- AltruismDocument3 pagesAltruismpeachybonyNo ratings yet
- Scan 24 Jul 23 10 15 38Document2 pagesScan 24 Jul 23 10 15 38peachybonyNo ratings yet
- 2018 Sem 1 QuestionsDocument10 pages2018 Sem 1 QuestionspeachybonyNo ratings yet
- DR Linda Molecular BiosDocument73 pagesDR Linda Molecular BiosValency BathoNo ratings yet
- Biotechnology8 q3 Mod2 Manipulation-of-Genetic-MaterialDocument16 pagesBiotechnology8 q3 Mod2 Manipulation-of-Genetic-Materialunc b50% (6)
- Biotechnology Principles and Processes - NotesDocument8 pagesBiotechnology Principles and Processes - NotesSquad 4 GamingNo ratings yet
- CyberbiosecurityDocument3 pagesCyberbiosecuritywatson191No ratings yet
- Bacterial Colony Isolation Using Serial Dilution TechniquesDocument2 pagesBacterial Colony Isolation Using Serial Dilution TechniquesAl Jay Mejos80% (5)
- Chang 2014 Social Isolation Induced Increase in NMDA Receptors in The Hippocampus Exacerbates Emotional Dysregulation in MiceDocument12 pagesChang 2014 Social Isolation Induced Increase in NMDA Receptors in The Hippocampus Exacerbates Emotional Dysregulation in MiceMarilyn GarciaNo ratings yet
- Effects of Chromolaena Odorata Leaf Meal SupplemenDocument10 pagesEffects of Chromolaena Odorata Leaf Meal SupplemenEmmanuel OwonaNo ratings yet
- Kharghar, Navi Mumbai - 410 210Document1 pageKharghar, Navi Mumbai - 410 210Ankit TiwariNo ratings yet
- Grammar 1 MinDocument8 pagesGrammar 1 Minlila bNo ratings yet
- Botany 1Document11 pagesBotany 1Richard NepomucenoNo ratings yet
- Hodges - The Guild Handbook of Scientific IllustrationDocument612 pagesHodges - The Guild Handbook of Scientific IllustrationGermán Huici Escribano100% (1)
- Nascimento Et Al. 2017 A Biologit S Guide To Bayesian Phylogenetic AnalysisDocument21 pagesNascimento Et Al. 2017 A Biologit S Guide To Bayesian Phylogenetic Analysisguilherme.rodrigues.bioNo ratings yet
- Practical Manual BIO 1141: University of Venda Department of Biological SciencesDocument42 pagesPractical Manual BIO 1141: University of Venda Department of Biological SciencesBelfry MantshwaneNo ratings yet
- قلق الموت من السرطانDocument212 pagesقلق الموت من السرطانBouchareb AhmedNo ratings yet
- B2 Reading 4Document4 pagesB2 Reading 4Herminio Garcia-RiañoNo ratings yet
- MicrobiologyDocument2 pagesMicrobiologyIrfan SalimNo ratings yet
- Synthesis and Characterization of Silica Microcapsules With Active Compounds 2% Chlorhexidine Using Sodium Alginate and Chitosan Coating As Medicament of Root Canal InfectionDocument7 pagesSynthesis and Characterization of Silica Microcapsules With Active Compounds 2% Chlorhexidine Using Sodium Alginate and Chitosan Coating As Medicament of Root Canal InfectionSEP-PublisherNo ratings yet
- Reincarnated As A Dragon Hatchling Volume 3Document290 pagesReincarnated As A Dragon Hatchling Volume 3Phil100% (1)
- 10-20 CAPTODAY HematologyAnalyzersDocument12 pages10-20 CAPTODAY HematologyAnalyzersalexandre larmagnacNo ratings yet
- Unit 2 Octopus VocabularyDocument6 pagesUnit 2 Octopus VocabularyBadass PolapainNo ratings yet
- 609 1121 1 SMDocument9 pages609 1121 1 SMpengembangan disbudparkabkediriNo ratings yet
- Anaphy Free Sample ExamDocument5 pagesAnaphy Free Sample ExamNurse UtopiaNo ratings yet
- 1erTareaMicroscopíasLópez Marmolejo Clere MishellDocument4 pages1erTareaMicroscopíasLópez Marmolejo Clere Mishellclere02marmolejoNo ratings yet
- Class 7 Science Sample Paper Term 2 Set 1 2021 2022Document2 pagesClass 7 Science Sample Paper Term 2 Set 1 2021 2022akashkushhNo ratings yet
- F3 End Term 2 Exams PDFDocument253 pagesF3 End Term 2 Exams PDFPAMAJA100% (1)
- Bioprospecting of Microbial Diversity Challenges and Applications in Biochemical Industry Agriculture and Environment Protection Pradeep Verma Maulin P Shah Full ChapterDocument52 pagesBioprospecting of Microbial Diversity Challenges and Applications in Biochemical Industry Agriculture and Environment Protection Pradeep Verma Maulin P Shah Full Chapterkristan.wade654100% (5)
- 2210030313FC Apipah ApriantiDocument1 page2210030313FC Apipah Apriantitomo bukakaNo ratings yet
- DescargaDocument22 pagesDescargaKemnet Aguirre ValenzuelaNo ratings yet