Professional Documents
Culture Documents
Gas Laws & Properties PRACTICE (1)_240430_173237
Gas Laws & Properties PRACTICE (1)_240430_173237
Uploaded by
actuallynaomixCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gas Laws & Properties PRACTICE (1)_240430_173237
Gas Laws & Properties PRACTICE (1)_240430_173237
Uploaded by
actuallynaomixCopyright:
Available Formats
Practice Worksheet: “Gas: Properties and Laws”
Name:
Class: Date:
Content Objective:
• To understand the contributions of Charles, Boyle, and Gay-Lussac to the field of science and
explore some key scientific vocabulary.
Language Objective:
• To strengthen reading and writing students' ability and to communicate about scientific principles
effectively.
Part 1: Fill in the blanks.
Complete the spaces below with words from the box. Be careful, there is one extra word you do not
need to use!
1) Boyle's Law explains the relationship between the pressure and
________________________of a gas when the temperature is kept constant.
2) Charles' Law describes how gases tend to expand when ________________________
increases.
3) The law that states the volume of a gas is directly proportional to its temperature is named
after ________________________
4) Gay-Lussac's Law focuses on the relationship between the pressure and
________________________of a gas at a constant volume.
Part 2: Multiple Choice.
Read each statement carefully and then circle the correct answer:
1) Who is known for stating that the pressure of a given mass of gas is inversely proportional to
its volume at constant temperature?
A) Charles
B) Boyle
C) Gay-Lussac
D) all of the above
2) Boyle's Law is related to the behavior of gases under variations of:
A) volume
B) pressure
C) temperature
D) density
3) Which scientist's law involves the relationship between the pressure and temperature of a gas
at constant volume?
A) Charles
B) Boyle
C) Gay-Lussac
D) none of them
Profesor(a): Vera Callejas
Curso: 7th B
Asignatura: Natural Science
4) According to Charles' Law, what happens to the volume of a gas if its temperature decreases?
A) Increases
B) Stays constant
C) Decreases
D) Fluctuates
5) Gay-Lussac's Law states that the pressure of a gas is directly proportional to its temperature if
the volume remains:
A) the same
B) decreases
C) increases
D) fluctuates
Part 3: Vocabulary
Write the definition of the following concepts and complement the information with an example (the
example can be in words or a drawing)
Concept Definition Example (write it or draw it)
Compression
Diffusion
Temperature
Volume
Part 5: Critical thinking
1) A container of gas has a volume of 500cm. at a pressure of 800atm. If the volume is reduced to
250cm. while the temperature remains constant, what will the new pressure be?
2) A gas in a closed container has an initial pressure of 100atm. at a temperature of 20°C. If the
temperature is increased to 50°C, what will be the new pressure if the volume remains constant?
3) A gas in a container has an initial volume of 2L at a temperature of 25°C. If the temperature is
increased to 50°C, what will the new volume be if the pressure remains constant?
Profesor(a): Vera Callejas
Curso: 7th B
Asignatura: Natural Science
You might also like
- Boyle's Law22 Lesson PlanDocument3 pagesBoyle's Law22 Lesson PlanMontesa Allana Ea82% (17)
- Pneumatics Systems: Ing. José de Jesús Mendoza OsorioDocument9 pagesPneumatics Systems: Ing. José de Jesús Mendoza Osoriojox1106No ratings yet
- Chem M9 Gas LawsDocument25 pagesChem M9 Gas LawsMa Perpetua Bardelas BaldescoNo ratings yet
- Joseph Louis Gay-Lussac Absolute TemperatureDocument4 pagesJoseph Louis Gay-Lussac Absolute TemperatureMira VeranoNo ratings yet
- The Gas LawsDocument12 pagesThe Gas LawsesssvieeNo ratings yet
- General Chemistry 1 Week 5 6Document10 pagesGeneral Chemistry 1 Week 5 6Emmanuel ValenzuelaNo ratings yet
- Gas LawsDocument27 pagesGas LawsGenesis Ng0% (3)
- Science 10 - Module Q4 SDO Malabon CityDocument29 pagesScience 10 - Module Q4 SDO Malabon CityHanna AngelaNo ratings yet
- Science-10 Q4 Mod1 Wk-1-2 ADM - EditedDocument19 pagesScience-10 Q4 Mod1 Wk-1-2 ADM - EditedMariah Paz Cadaoas100% (1)
- II. Direction: Balance The Following Chemical EquationsDocument3 pagesII. Direction: Balance The Following Chemical EquationsHera S LinduganNo ratings yet
- II. Direction: Balance The Following Chemical EquationsDocument3 pagesII. Direction: Balance The Following Chemical EquationsHera S LinduganNo ratings yet
- Eastern Visayas State University: Education DepartmentDocument3 pagesEastern Visayas State University: Education DepartmentMichelle EscalienteNo ratings yet
- Che 205 Practice Questions 1Document9 pagesChe 205 Practice Questions 1Sunday EdemaNo ratings yet
- SCIENCE10 Q4W1 v2Document16 pagesSCIENCE10 Q4W1 v2Trixie EuniceNo ratings yet
- Gas LawsDocument80 pagesGas LawsChennille Ann Bleu GundayaoNo ratings yet
- Gas Laws 1Document115 pagesGas Laws 1Jhamia Cruz EstradaNo ratings yet
- Science 10 Module 4Q 2023Document26 pagesScience 10 Module 4Q 2023Arlene SantosNo ratings yet
- Unittest 4Document1 pageUnittest 4Jomar DerayNo ratings yet
- Revised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarDocument11 pagesRevised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarSaysain UkayNo ratings yet
- BOYLE'S LAW Group 1Document13 pagesBOYLE'S LAW Group 1Erich UntalanNo ratings yet
- Science 10 Q4 Module 1Document29 pagesScience 10 Q4 Module 1Maki Tuna100% (2)
- 4th Periodic Exam SCIENCE10Document5 pages4th Periodic Exam SCIENCE10Lily Mae MontalbanNo ratings yet
- M2 For Printing PDFDocument24 pagesM2 For Printing PDFcute chubbitNo ratings yet
- Gas Laws: Solids LiquidsDocument2 pagesGas Laws: Solids LiquidsRaymond CabanillaNo ratings yet
- Science 10 Las 4-1Document5 pagesScience 10 Las 4-1Michael TuyayNo ratings yet
- 4th Quarter Test Science 10Document5 pages4th Quarter Test Science 10Precy CoNo ratings yet
- LAS in Science 10 - Week 4Document2 pagesLAS in Science 10 - Week 4Allynn JunioNo ratings yet
- G10 Q4 Melc 21Document16 pagesG10 Q4 Melc 21valdellondonjoseph1No ratings yet
- 3-4 Gas Laws Int - Reader - Study - Guide PDFDocument6 pages3-4 Gas Laws Int - Reader - Study - Guide PDFel graceNo ratings yet
- Final Fluids Design ExpeDocument13 pagesFinal Fluids Design ExpekrakatuaNo ratings yet
- 2 K Aj 8 Zroh YCA7 Ixv 6 Ta UB4 Q 83 LM TMSK CXNW Mu X3 IDocument5 pages2 K Aj 8 Zroh YCA7 Ixv 6 Ta UB4 Q 83 LM TMSK CXNW Mu X3 IMrlybucks Twitch Or YTNo ratings yet
- Test Ch. 12 (The Gas Laws) PracticeDocument4 pagesTest Ch. 12 (The Gas Laws) Practiceliza1207No ratings yet
- G10 Charles Law 2Document16 pagesG10 Charles Law 2Ciara BellaNo ratings yet
- ASMEPPS Reviewer Chemistry 1Document2 pagesASMEPPS Reviewer Chemistry 1Morphetz ErtsNo ratings yet
- 2.10 Gizmo Charles's Law Part BDocument5 pages2.10 Gizmo Charles's Law Part Bhihi70547No ratings yet
- Aynchronous Task 3Document3 pagesAynchronous Task 3galveznyebessolanaNo ratings yet
- Science 10 Q4 Module 2Document13 pagesScience 10 Q4 Module 2Dennis Douglas Alo Jr.No ratings yet
- Student Exploration: Boyle's Law and Charles' LawDocument8 pagesStudent Exploration: Boyle's Law and Charles' LawjzbNlka0% (1)
- Science 10 Q4 Mod1 Behavior of Gases FinalDocument19 pagesScience 10 Q4 Mod1 Behavior of Gases FinalBitancor JemimaNo ratings yet
- Student Exploration: Boyle's Law and Charles' LawDocument7 pagesStudent Exploration: Boyle's Law and Charles' Lawapi-280439402No ratings yet
- Science 10 - Week 27Document3 pagesScience 10 - Week 27Mira VeranoNo ratings yet
- UP2 - Ch2. The Kinetic Theory of GasesDocument42 pagesUP2 - Ch2. The Kinetic Theory of Gasesdarnit2703No ratings yet
- 1 Science 10 Q4 Charles Law Sy2324Document2 pages1 Science 10 Q4 Charles Law Sy2324lanstubang69No ratings yet
- Science Q4 Summative TestDocument5 pagesScience Q4 Summative Testlester bessittNo ratings yet
- Boyles Law Lesson PlanDocument2 pagesBoyles Law Lesson PlanFany Fabia60% (5)
- Nature-Of-Gases-Compilation - Montallana and MorenoDocument4 pagesNature-Of-Gases-Compilation - Montallana and MorenoRhave MorenoNo ratings yet
- LESSON PLAN IN SCIENCE 10: Avogadro's LawDocument3 pagesLESSON PLAN IN SCIENCE 10: Avogadro's Lawrigie.divinagraciaNo ratings yet
- Behavior of Gases 4th M1Document24 pagesBehavior of Gases 4th M1April Rose Caquilala GalauraNo ratings yet
- Pretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerDocument6 pagesPretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerClarence Mike BorjaNo ratings yet
- 5-6 G10 ScienceDocument2 pages5-6 G10 Scienceisaacsamuel229No ratings yet
- Quiz - Chapter 6Document5 pagesQuiz - Chapter 6dNo ratings yet
- Q4 Summative-Test M1-M2 052223Document11 pagesQ4 Summative-Test M1-M2 052223Shanelle shaine ParreñoNo ratings yet
- C.O 4th QuarterDocument7 pagesC.O 4th QuarterOdessa Niña Pilapil Fernandez100% (1)
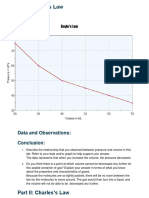
- Data and Observations: ConclusionDocument2 pagesData and Observations: ConclusionStephany LeviNo ratings yet
- Austin, Vinny, Steven - Combined Gas Law PresentationDocument24 pagesAustin, Vinny, Steven - Combined Gas Law PresentationAustin MaNo ratings yet
- Worksheet Boyles LawDocument4 pagesWorksheet Boyles Lawmaricore montesNo ratings yet
- Assignment: Science IV: 1.) Boyle's LawDocument6 pagesAssignment: Science IV: 1.) Boyle's LawIrish WahidNo ratings yet
- ICSE Selina Solution For Class 9 Chemistry Chapter 7 Exercise QuestionsDocument31 pagesICSE Selina Solution For Class 9 Chemistry Chapter 7 Exercise QuestionsAmit100% (1)
- Global Warming Temperatures and Projections: As Related to CO2 and H2O Absorptions, H2O Evaporation, and Post-Condensation ConvectionFrom EverandGlobal Warming Temperatures and Projections: As Related to CO2 and H2O Absorptions, H2O Evaporation, and Post-Condensation ConvectionNo ratings yet
- Thermodynamics ReviewerDocument2 pagesThermodynamics ReviewerRogelioB.AlobII100% (1)
- Chemical Equilibrium Mixture Computations For Energetic Material Combustion in Closed VesselsDocument9 pagesChemical Equilibrium Mixture Computations For Energetic Material Combustion in Closed VesselssirusNo ratings yet
- Unit-1 Chemistry (Matter) Lesson 1-A Properties of Matter-Solid, Liquid, and Gas (Grade 3) ObjectivesDocument16 pagesUnit-1 Chemistry (Matter) Lesson 1-A Properties of Matter-Solid, Liquid, and Gas (Grade 3) Objectiveshaizelle resmaNo ratings yet
- BHEL Centrifugal CompressorDocument6 pagesBHEL Centrifugal CompressorJitendra JaiswalNo ratings yet
- CPP Pocket ManualDocument180 pagesCPP Pocket ManualdevagiriNo ratings yet
- 1 Ultrasonic Manuals and InstructionsDocument142 pages1 Ultrasonic Manuals and InstructionsMartin Alejandro SilvaNo ratings yet
- Relationship BTWN Pressure & VolumeDocument1 pageRelationship BTWN Pressure & Volumeareyouthere92No ratings yet
- BSC PhysicsDocument92 pagesBSC PhysicsHarry-pearceNo ratings yet
- Certificate: Well Test Analysis For Gas Condensate ReservoirDocument19 pagesCertificate: Well Test Analysis For Gas Condensate Reservoiryash chavanNo ratings yet
- PE363 - Chapter 1Document49 pagesPE363 - Chapter 1abduNo ratings yet
- Particles in Solids, Liquids, and GasesDocument6 pagesParticles in Solids, Liquids, and Gasesjhunior carlos eduardo gamboa herreraNo ratings yet
- Parker Space Quick DisconnectDocument8 pagesParker Space Quick DisconnectAdnan DincerNo ratings yet
- 2016 Reshuffling Test Mains Paper With SolutionsDocument25 pages2016 Reshuffling Test Mains Paper With SolutionsAshutosh GourNo ratings yet
- Division 2.2: Linde Gas LLC P.O. Box 94737 Cleveland, Ohio 44101Document4 pagesDivision 2.2: Linde Gas LLC P.O. Box 94737 Cleveland, Ohio 44101Ruang RenungNo ratings yet
- Cambridge ChemistryDocument100 pagesCambridge ChemistryBraweet SapkotaNo ratings yet
- Compressor Training ModuleDocument29 pagesCompressor Training ModuleHyundianto Ag100% (1)
- S2 PHYSICS Unit-3 & 6 & 9 Revision NoteDocument16 pagesS2 PHYSICS Unit-3 & 6 & 9 Revision NoteNay Myo Lin (Kouji)No ratings yet
- Complete Chemistry For IGCSE Chapter 1Document20 pagesComplete Chemistry For IGCSE Chapter 1Hubbak Khan100% (5)
- Uranium Talent Search Examination (Utse) - 2013: Physics SectionDocument5 pagesUranium Talent Search Examination (Utse) - 2013: Physics SectionDebasis KarNo ratings yet
- GT Operation and Maintenance Consideration PDFDocument73 pagesGT Operation and Maintenance Consideration PDFThanapaet Rittirut100% (1)
- Compressible Fluids: 2004 Faith A. Morrison, All Rights ReservedDocument5 pagesCompressible Fluids: 2004 Faith A. Morrison, All Rights ReservedcoffewhoreNo ratings yet
- (Kids) Chemistry Science Experiments 2Document129 pages(Kids) Chemistry Science Experiments 2CNMarmo100% (3)
- Chemistry Objective NCERT Xtract WWW - examSAKHA.in PDFDocument781 pagesChemistry Objective NCERT Xtract WWW - examSAKHA.in PDFAshwath KuttuvaNo ratings yet
- Smart Helmet For Mining WorkersDocument7 pagesSmart Helmet For Mining WorkersIJRASETPublicationsNo ratings yet
- Factors Affecting SolubilityDocument2 pagesFactors Affecting SolubilityYuppie Raj100% (2)
- Foxtrot (4.5) For PresentationDocument45 pagesFoxtrot (4.5) For PresentationAnonymous b9iturNo ratings yet
- CH - 6 Saturated Oil ReservoirsDocument37 pagesCH - 6 Saturated Oil ReservoirszazoNo ratings yet
- H010920 MPFM PDFDocument2 pagesH010920 MPFM PDFFriday IjokgwungNo ratings yet
- SEM 3037E Tower Piping.Document52 pagesSEM 3037E Tower Piping.Kodali Naveen KumarNo ratings yet
- ExerciseDocument48 pagesExerciseYash MohtaNo ratings yet