Professional Documents
Culture Documents
2 Principles of Interchangeability
2 Principles of Interchangeability
Uploaded by
Nelson MoreriCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2 Principles of Interchangeability
2 Principles of Interchangeability
Uploaded by
Nelson MoreriCopyright:
Available Formats
Medicines Control Authority of
Zimbabwe
Regional Centre of Regulatory Excellence
(RCORE)
In
Medicine Evaluation & Registration
Bioequivalence Workshop
MCAZ Evaluations & Registration Division
15 – 19 October 2018
Protecting Your Right to Quality Medicines
Medicines Control Authority of
Zimbabwe
Bioequivalence Workshop
2. Principles of
Interchangeability &
Bioequivalence
MCAZ Evaluations & Registration Division
15 – 19 October 2018
Protecting Your Right to Quality Medicines
At the end of the session…
You should be able to:
1. Articulate what interchangeability is and how it
can be demonstrated
2. Explain the basis and requirements for
bioequivalence requirement for generic
products
3. Describe the approaches to demonstrate BE (in
vitro and in vivo)
4. Demonstrate the ability to interpret regulatory
requirements
Protecting Your Right to Quality Medicines
Introduction
• Bioavailability/ bioequivalence requirements for
generics is well-established over last 5-decades
• Limited experience & practice with
bioequivalence & WHO requirements for African
manufacturers and regulators
• Few NMRAs adapted / adopted WHO BA/BE
Guidelines
- First WHO BA/BE Guideline published in 1996 – WHO
Technical Report Series # 863, Annex 9
Protecting Your Right to Quality Medicines
Brief introduction to bioequivalence
• Multi-source (generic) drug products need to conform
to the same standards of quality, efficacy and safety
required of the originator's (innovator/brand)
products.
• A reasonable assurance must be provided that they
are, as intended, clinically interchangeable with
innovator product or acceptable comparator products
• Pharmaceutically equivalent multi-source
pharmaceutical products must be shown to be
therapeutically equivalent to one another in order to
be considered interchangeable
Protecting Your Right to Quality Medicines
Types of equivalence studies
Sensitivity to detect differences
• Pharmacokinetic studies
• Pharmacodynamic studies and
• Comparative clinical trials
• In vitro studies
- In vitro studies are rarely validated as surrogate of BE
- But if validated, they are the most sensitive
- BCS Biowaiver, IVIVC,
Protecting Your Right to Quality Medicines
Bioavailability / Bioequivalence
• Bioavailability of a drug is:
- Rate and extent to which the API is absorbed
from a pharmaceutical form and reaches the
systematic circulation intact
• Two pharmaceutical products are bioequivalent if they are:
- Pharmaceutically equivalent or pharmaceutical alternatives, and
their bioavailabilities, in terms of peak (Cmax and Tmax) and total
exposure (area under the curve (AUC)) after administration of the
same molar dose under the same conditions, are similar to such a
degree that their effects can be expected to be essentially the
same.
Protecting Your Right to Quality Medicines
A Bit of History
Hatch-Waxman
Act of 1984
1980s
GL Amidon,
1995
WHO TRS
863, 1996 1990s
FDA BCS
Guideline, 2000
WHO TRS 2000s
937, 2006
Protecting Your Right to Quality Medicines
A Bit of History cont’d,
EU BA/BE
Guideline
2010
FDA (draft)
Updated
Guidelines
WHO TRS
2015
992, 2015
Convergence of regulatory
requirements: BCS biowaivers
Class I & III
Protecting Your Right to Quality Medicines
• All pharmaceutical products,
including multisource
products, should be used in
a country only after
approval by the national or
regional authority.
Regulatory authorities
should require the
documentation of a
multisource pharmaceutical
product to meet the
following:
• –– GMP;
• –– QC specifications;
• –– pharmaceutical product
interchangeability
Protecting Your Right to Quality Medicines
Generic product - drug application
Quality Quality
Pre-clinical Pre-clinical
Innovator Generic
-Efficacy/Safety product
product Clinical
Clinical application
application Pharmacokinetics
BE studies
Pharmacovig
Pharmacov
ilance igilance
Protecting Your Right to Quality Medicines
Requirements for Generic medicines
Lembit Rago and Budiono Santoso, Chapter 6: Drug Regulation: History, Present and Future: In Drug Benefits and Risks: International Textbook of
Clinical Pharmacology, revised 2nd edition, Edited by C.J. van Boxtel, B. Santoso and I.R. Edwards. IOS Press and Uppsala Monitoring Centre, 2008.
Protecting Your Right to Quality Medicines
Principles of interchangeability
• Bioequivalence is direct proof that the generic
product can be used for new patients with the
same benefit and risk:
- “Prescribability” (new prescriptions, without any adjustment in dose
or other additional therapeutic monitoring)
• A patient already under treatment with the
innovator product can generally be switched to
the generic product: “Switchability” (additional
prescriptions)
• Bioequivalence is also generally considered as
indirect proof of “switchability” between generics
/ multisource products
Protecting Your Right to Quality Medicines
Therapeutic equivalence vs.
Bioequivalence
• Therapeutic equivalence can be assured when the
generic product is both pharmaceutically
equivalent/alternative and bioequivalent.
• Bioequivalence based on blood level determination
of Cmax and AUC has become the most commonly
used and successful biomarker for safety and
efficacy of the drug product.
Protecting Your Right to Quality Medicines
Therapeutic equivalence
• Direct practical demonstration of therapeutic
equivalence in a clinical study usually requires
large numbers of patients.
• Such studies in humans can be financially
daunting, are often unnecessary and may be
unethical
Protecting Your Right to Quality Medicines
Scope of the Bioequivalence Guideline
• This guidance is generally applicable to orally
administered multisource products, as well as
to non-orally administered pharmaceutical
products for which systemic exposure
measures are suitable for documenting
bioequivalence (e.g. transdermal delivery
systems and certain parenteral, rectal and
nasal pharmaceutical products).
Protecting Your Right to Quality Medicines
Scope of the Bioequivalence Guideline
• Systemic action
– Oral Immediate Release or Modified Release Products
– Transdermal products with systemic action
– Certain parental products (e.g. prolonged release),
– Rectal, nasal, etc. with systemic effect
• Local action
– Inhalation products
• For systemic safety (FDA, Canada, EU)
• Lung deposition (total and pattern of deposition in EU) as surrogate of efficacy
– Nasal products
• For systemic safety (nasal suspensions in FDA draft guidance)
– Gastrointestinal tract (certain drug specific guidance in FDA webpage)
• For systemic safety + in vitro dissolution
Protecting Your Right to Quality Medicines
Biowaiver
• Proportional formulations
- Bioequivalence has been shown with one strength (most sensitive
strength)
- Same manufacturing process
- Similar dissolution profile
• Certain dosage forms (e.g. oral solutions)
- For some classes of product, including – most evidently – parenteral
formulations of highly water-soluble compounds, interchangeability is
adequately assured by implementation of GMP and evidence of
conformity with relevant pharmacopoeial specifications.
• BCS Biowaivers
- In selected cases, in vitro comparison of dissolution profile of the
multisource product with that of the comparator product, or dissolution
studies, may be sufficient to provide indication of equivalence
Protecting Your Right to Quality Medicines
Biowaiver for certain dosage forms
Pharmaceutical equivalence may be enough in
case of:
– Oral aqueous solutions (excluding active excipients)
– Aqueous Intravenous solutions (similar excipients)
– Aqueous Intramuscular / Subcutaneous solutions (similar excipients and
viscosity)
– Oily Intramuscular / Subcutaneous solutions (same oil)
– Aqueous Optic / Ophthalmic solutions
– Cutaneous solutions (if same Qualitative and Quantitative composition)
– Nasal Solutions (if same quali and quanti + in vitro testing for device
performance)
– Powders / Granules for reconstitution as solution
– Gases
Protecting Your Right to Quality Medicines
When equivalence studies are not necessary (a)
• The following types of multisource pharmaceutical product are
considered to be equivalent without the need for further documentation:
• (a) when the pharmaceutical product is to be administered parenterally
(e.g. intravenously, subcutaneously or intramuscularly) as an aqueous
solution containing the same API in the same molar concentration as the
comparator product and the same or similar excipients in comparable
concentrations as in the comparator product.
• Certain excipients (e.g. buffer, preservative and antioxidant) may be
different provided it can be shown that the change(s) in these excipients
would not affect the safety and/or efficacy of the pharmaceutical product
Protecting Your Right to Quality Medicines
Scenario b
• (b) when pharmaceutically equivalent products
are solutions for oral use (e.g. syrups, elixirs and
tinctures), contain the API in the same molar
concentration as the comparator product, and
contain essentially the same excipients in
comparable concentrations.
• Excipient(s) known to affect gastrointestinal (GI)
transit, GI permeability and hence absorption or
stability of the API in the GI tract should be
critically reviewed;
Protecting Your Right to Quality Medicines
Scenario c - d
• (c) when pharmaceutically equivalent
products are in the form of powders for
reconstitution as a solution and the resultant
solution meets either criterion (a) or criterion
(b) above;
• (d) when pharmaceutically equivalent
products are gases;
Protecting Your Right to Quality Medicines
Scenario e
• (e) when pharmaceutically equivalent products
are optic or ophthalmic products prepared as
aqueous solutions and contain the same API(s) in
the same molar concentration and essentially the
same excipients in comparable concentrations.
• Certain excipients (e.g. preservative, buffer,
substance to adjust tonicity or thickening agent)
may be different provided their use is not
expected to affect safety and/or efficacy of the
product
Protecting Your Right to Quality Medicines
Scenario f
• (f) when pharmaceutically equivalent
products are topical products prepared as
aqueous solutions and contain the same
API(s) in the same molar concentration and
essentially the same excipients in comparable
concentrations;
Protecting Your Right to Quality Medicines
Scenario g
• (g) when pharmaceutically equivalent products
are aqueous solutions for nebulizer inhalation
products or nasal sprays, intended to be
administered with essentially the same device,
and contain the same API(s) in the same
concentration and essentially the same excipients
in comparable concentrations.
• The pharmaceutical product may include
different excipients provided their use is not
expected to affect safety and/or efficacy of the
product.
Protecting Your Right to Quality Medicines
Scenarios (cont)
• For situations (b), (c), (e), (f) and (g) above, it is
incumbent upon the applicant to demonstrate that the
excipients in the pharmaceutically equivalent product
are essentially the same and in concentrations
comparable to those in the comparator product or,
where applicable (i.e. (e) and (g)), that their use is not
expected to affect the safety and/or efficacy of the
product.
• In the event that this information cannot be provided
by the applicant and the drug regulatory authority
does not have access to the relevant data, it is
incumbent upon the applicant to perform appropriate
studies to demonstrate that differences in excipients or
devices do not affect product performance.
Protecting Your Right to Quality Medicines
Bioequivalence cannot be assumed
• Pharmaceutical Equivalent Products
Pharmaceutical equivalence does not necessarily imply
therapeutic equivalence Test
Reference
• Differences in:
– Raw materials Possible Differences
• Drug (e.g. particle size, polymorphism, etc.)
• Excipients (e.g. grade) Drug particle size, ...
– Formulation / composition Excipients
• Q1 and Q2 (effect on in vivo dissolution and
absorption) Manufacturing process
– Manufacturing process (e.g. dry vs. wet granulation)
Equipment
– Equipment
– Site of Manufacturing Site of manufacture
– Batch size
Batch size ….
• May affect the bioavailability of pharmaceutical
equivalents or pharmaceutical alternatives.
• Then, bioequivalence has to be demonstrated Documented Bioequivalence
= Therapeutic Equivalence
Protecting Your Right to Quality Medicines
Bioequivalence / Comparative
Bioavailability is required
In vivo documentation of equivalence is needed
when there is a risk that possible differences in
bioavailability may result in therapeutic
inequivalence.
When the innovator develops a new formulation
– During the drug-development process (before approval)
– As a variation of the marketed product after approval
When the innovator develops a new dosage form
– Line extension
When a multisource (generic) product is applied
for marketing authorisation
Protecting Your Right to Quality Medicines
Interchangeability
The concept of interchangeability includes the
equivalence of the dosage form as well as of the
indications and instructions for use.
– If BE is shown, it is equivalent in all indications if used
according to the instructions of use.
– Some indications may be protected by patent
– Interchangeability
• If both products in the same dosage form in some countries (e.g. USA)
• Between capsules and tablets (e.g. Canada)
• All oral immediate release dosage forms (e.g. EU)
Protecting Your Right to Quality Medicines
Summary
• Bioequivalence ensures a similar
biopharmaceutical quality or in vivo
performance.
– This may require more than one study
• Fasted state
• Fed state
Then, it is assumed that the multisource product can be used
instead of the reference in all type of patients
– Elderly, Children
– Renal / Hepatic impairment.
Protecting Your Right to Quality Medicines
You might also like
- Medication Safety For Medicines: Look-Alike, Sound-AlikeDocument36 pagesMedication Safety For Medicines: Look-Alike, Sound-AlikesteefhNo ratings yet
- Hughes PatriciaDocument60 pagesHughes PatriciaNing KevinNo ratings yet
- 2 Principles of InterchangeabilityDocument30 pages2 Principles of InterchangeabilityNelson MoreriNo ratings yet
- Bioequivalence StudiesDocument67 pagesBioequivalence StudiesAidee SmithNo ratings yet
- Bioequivalence Requirements in Various Global Jurisdictions-Springer International Publishing (2017)Document348 pagesBioequivalence Requirements in Various Global Jurisdictions-Springer International Publishing (2017)Solomon100% (1)
- Under Guidance of Mr. Mali K.K. (Assistant Professor) : 1 Yspm, YtcDocument41 pagesUnder Guidance of Mr. Mali K.K. (Assistant Professor) : 1 Yspm, YtcSabiruddin Mirza DipuNo ratings yet
- Commentary Global Harmonization of Comparator Products For Bioequivalence StudiesDocument4 pagesCommentary Global Harmonization of Comparator Products For Bioequivalence StudiesBilal AbbasNo ratings yet
- Multisource Pharmaceutical Products Guidelines Registration Annex7Document44 pagesMultisource Pharmaceutical Products Guidelines Registration Annex7hernandez rebecaNo ratings yet
- Abbreviated New Drug ApplicationDocument20 pagesAbbreviated New Drug Applicationmanisha sainiNo ratings yet
- Bio Similar S GuidanceDocument6 pagesBio Similar S Guidancegulafsha1No ratings yet
- Bioavailability and BoequivalenceDocument47 pagesBioavailability and BoequivalenceSilvyNo ratings yet
- Bioequivalence: Faculty of Pharmacy, Nursing and Health Professions Master in Industrial Pharmaceutical TechnologyDocument16 pagesBioequivalence: Faculty of Pharmacy, Nursing and Health Professions Master in Industrial Pharmaceutical TechnologyMayson BaliNo ratings yet
- Abbreviated New Drug AppliationDocument21 pagesAbbreviated New Drug AppliationShubham SuleNo ratings yet
- Genericdrugs 0Document43 pagesGenericdrugs 0Ahmed AliNo ratings yet
- Biosimilars InterchangeabilityDocument2 pagesBiosimilars InterchangeabilityPORTETHONo ratings yet
- Bioequivalence: Faculty of Pharmacy, Nursing and Health Professions Master in Industrial Pharmaceutical TechnologyDocument15 pagesBioequivalence: Faculty of Pharmacy, Nursing and Health Professions Master in Industrial Pharmaceutical TechnologyMayson Bali100% (1)
- 8 Ba Be FdaDocument36 pages8 Ba Be Fdaraju narayana padalaNo ratings yet
- Unit 4 - Medical (Pharmaceutical) TerminologiesDocument79 pagesUnit 4 - Medical (Pharmaceutical) TerminologiesShashidharan MenonNo ratings yet
- Benefits and Risks To Biosimilars: From A Patient Perspective?Document15 pagesBenefits and Risks To Biosimilars: From A Patient Perspective?drtanoyboseNo ratings yet
- Riesgo MicrobiologicoDocument47 pagesRiesgo MicrobiologicocarbouNo ratings yet
- Guidance On Registration of Similar Biological Products in SingaporeDocument13 pagesGuidance On Registration of Similar Biological Products in SingaporeWilliam ChandraNo ratings yet
- Biosimilars: The Future of Prescribing Biological MedicinesDocument35 pagesBiosimilars: The Future of Prescribing Biological MedicinesFatina HatoumNo ratings yet
- ECA Combination Products 2024 Live Online TrainingDocument4 pagesECA Combination Products 2024 Live Online Trainingmichael.lichfieldNo ratings yet
- Biowaivers Criteria RequirementsDocument11 pagesBiowaivers Criteria RequirementsSaravanan AnnamalaiNo ratings yet
- 3 General Introduction in BE and Study Design-Botswana 2019Document56 pages3 General Introduction in BE and Study Design-Botswana 2019Nelson MoreriNo ratings yet
- Lecture 10 - PHR514-Pharmacy Law and Regulatory AffairsDocument26 pagesLecture 10 - PHR514-Pharmacy Law and Regulatory AffairsNeymar ShuvoNo ratings yet
- The Orange BookDocument6 pagesThe Orange Book50KMKDIVYA RAJPALNo ratings yet
- AmlodipinebesylatepaperDocument6 pagesAmlodipinebesylatepaperRakesh RauniyarNo ratings yet
- THEORANGEBOOKDocument6 pagesTHEORANGEBOOKMashiko DakhundaridzeNo ratings yet
- Biosimilares 2.0Document8 pagesBiosimilares 2.0Carlos Julio Nova LopezNo ratings yet
- (첨부 1) Guideline for Registration of Medicines (FMHACA)Document8 pages(첨부 1) Guideline for Registration of Medicines (FMHACA)Talha MuhammadNo ratings yet
- KJBVGGVVVDocument10 pagesKJBVGGVVVSuvojit BasakNo ratings yet
- Accelerated Stability Test of Ferrous Sulfate Tablets in WaterDocument27 pagesAccelerated Stability Test of Ferrous Sulfate Tablets in WaterJeyma DacumosNo ratings yet
- Presentation Interchangeability Generics - enDocument20 pagesPresentation Interchangeability Generics - enSufian MohammedNo ratings yet
- Overview of Complex Generics Regulatory Perspective On BioequivalenceDocument41 pagesOverview of Complex Generics Regulatory Perspective On BioequivalenceshrikantNo ratings yet
- BioequivalenceDocument30 pagesBioequivalenceTejas PatelNo ratings yet
- BE and Drug Product AssesstmentDocument20 pagesBE and Drug Product AssesstmentRenaldy NongbetNo ratings yet
- Note For Guidance On The Investigation of Bioavailability & BioequivalenceDocument19 pagesNote For Guidance On The Investigation of Bioavailability & BioequivalenceAhmed AliNo ratings yet
- Regulatory Requirement of Similar Biologic For Marketing AuthorizationDocument19 pagesRegulatory Requirement of Similar Biologic For Marketing AuthorizationSatheesh SachinNo ratings yet
- EN Patient QA On Biosimilars July 2016 FINAL For Publication Branded v3Document9 pagesEN Patient QA On Biosimilars July 2016 FINAL For Publication Branded v3Carmen Alerany PardoNo ratings yet
- Bioavailability & Bioequivalence Pharmacokinetic PrinciplesDocument24 pagesBioavailability & Bioequivalence Pharmacokinetic PrinciplesParthMairNo ratings yet
- Quality, QC, QM, Quality Assurance - 11Document32 pagesQuality, QC, QM, Quality Assurance - 11Muhammad Shahedul IslamNo ratings yet
- Fast Facts: Biosimilars in Hematology and Oncology: Biologics and biosimilars - getting decisions rightFrom EverandFast Facts: Biosimilars in Hematology and Oncology: Biologics and biosimilars - getting decisions rightNo ratings yet
- 3 2809 Ws Setting Pharmacopoeial Standards For Biotherapeutic Products-MorningDocument70 pages3 2809 Ws Setting Pharmacopoeial Standards For Biotherapeutic Products-MorningyolsuzzNo ratings yet
- PQRI - Biosimilar USADocument27 pagesPQRI - Biosimilar USANgoc Sang HuynhNo ratings yet
- International PV Lec 4Document26 pagesInternational PV Lec 4Eman AhmedNo ratings yet
- Comparing Generic and Innovator Drugs A Review ofDocument16 pagesComparing Generic and Innovator Drugs A Review ofPrachi PandeyNo ratings yet
- Generics and Rational Use of Medicines PresentationDocument44 pagesGenerics and Rational Use of Medicines PresentationAngelica BolosNo ratings yet
- Pharmacovigilance-Related Topics Lec 2 CpyDocument22 pagesPharmacovigilance-Related Topics Lec 2 CpyEman AhmedNo ratings yet
- Guidance: in Vivo BioequivalenceDocument14 pagesGuidance: in Vivo BioequivalenceAnonymous Se5IdneSpNo ratings yet
- Difference Between Bioavailability and BioequivalenceDocument11 pagesDifference Between Bioavailability and Bioequivalenceprakash deshmukhNo ratings yet
- Generic Medicines: Interchangeability of WHO-prequalified GenericsDocument6 pagesGeneric Medicines: Interchangeability of WHO-prequalified GenericsGuide LPNo ratings yet
- ANDADocument28 pagesANDAShubham SuleNo ratings yet
- Biosimilars in Ophthalmology: A ReviewDocument11 pagesBiosimilars in Ophthalmology: A Reviewindex PubNo ratings yet
- EMEA - BioSimilar Guidelines 2Document32 pagesEMEA - BioSimilar Guidelines 2Shrinivas TamaskarNo ratings yet
- 2011 - The Biowaiver Procedure - Its Application To Antituberculosis Products in The WHO Precualification ProgrammeDocument9 pages2011 - The Biowaiver Procedure - Its Application To Antituberculosis Products in The WHO Precualification ProgrammePedro Marcelo Alva PlasenciaNo ratings yet
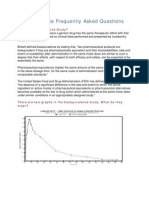
- Bioequivalence Frequently Asked Questions: What Is Bioequivalence Study?Document4 pagesBioequivalence Frequently Asked Questions: What Is Bioequivalence Study?Ahmed HasanNo ratings yet
- Jurnal AmlodipinDocument4 pagesJurnal AmlodipindidiisafitriNo ratings yet
- Bioequivalence and ADMEDocument18 pagesBioequivalence and ADMEIvory DianeNo ratings yet
- Craneetal 2019Document16 pagesCraneetal 2019บอส เลิศเกียรติรัชตะNo ratings yet
- Guideline For Bioequivalence Studies of Generic ProductsDocument23 pagesGuideline For Bioequivalence Studies of Generic ProductschetanjmistryNo ratings yet
- 4 Principles of GCP MlfDocument28 pages4 Principles of GCP MlfNelson MoreriNo ratings yet
- 11 Dissolution & biowaiversDocument72 pages11 Dissolution & biowaiversNelson MoreriNo ratings yet
- 1 Intro to the Reg of medicines (1)Document46 pages1 Intro to the Reg of medicines (1)Nelson MoreriNo ratings yet
- Regulatory Affairs Article 1Document1 pageRegulatory Affairs Article 1Nelson MoreriNo ratings yet
- Stok 23 JanDocument196 pagesStok 23 JanNando AswinNo ratings yet
- Joneckis FDA Product Regulation ALLDocument130 pagesJoneckis FDA Product Regulation ALLwagnerthales23No ratings yet
- Research Methodology PraveenDocument12 pagesResearch Methodology PraveenpraveenNo ratings yet
- Instructions - For - Use ValtocoDocument2 pagesInstructions - For - Use Valtocofreebass08No ratings yet
- Drug Education and Vice Control: Reviewer Notes inDocument10 pagesDrug Education and Vice Control: Reviewer Notes inJolly BelleNo ratings yet
- Mechanism of Action of QuetiapineDocument4 pagesMechanism of Action of QuetiapineThoha AlbaarNo ratings yet
- Daftar Obat Aman Dan Berbahaya Untuk Ibu Hamil DanDocument4 pagesDaftar Obat Aman Dan Berbahaya Untuk Ibu Hamil Daninne_fNo ratings yet
- Drug Design: Optimizing Target InteractionsDocument69 pagesDrug Design: Optimizing Target InteractionsDRx Ijajul HussainNo ratings yet
- Format Lplpo 2023 New DinasDocument65 pagesFormat Lplpo 2023 New DinasFatyun ailatatNo ratings yet
- Lesson 1 Prescription and Medication OrderDocument30 pagesLesson 1 Prescription and Medication OrderAngelica GomezNo ratings yet
- NCM 106 (PHARMA) - Lec 19 To 28Document18 pagesNCM 106 (PHARMA) - Lec 19 To 28Aine Jermaine GatchalianNo ratings yet
- Daftar PustakaDocument7 pagesDaftar PustakaYazuko RizuyaNo ratings yet
- PSYCHOPHARMACOLOGYDocument2 pagesPSYCHOPHARMACOLOGYJulia Rae Delos SantosNo ratings yet
- BSP2ADocument10 pagesBSP2AMA. CRISTHAN SALCEDONo ratings yet
- Resume Template PharmacistDocument1 pageResume Template PharmacistHammad AslamNo ratings yet
- Parenteral PreparationDocument16 pagesParenteral PreparationSyed R ShohanNo ratings yet
- PharJur Ass#2Document2 pagesPharJur Ass#2Reina Carissa RodilNo ratings yet
- Group Assignment Intro To PharmaDocument17 pagesGroup Assignment Intro To PharmaIbsa Ahmed Yusuf PharmacistNo ratings yet
- 160 مصنع تايلند حاصلة على الgmpDocument70 pages160 مصنع تايلند حاصلة على الgmpخبراء التصنيع الدوائي-اليمنNo ratings yet
- Xylocaine Dentaland AdrenalineDocument3 pagesXylocaine Dentaland AdrenalinePir Majid ShahNo ratings yet
- DDS NotesDocument31 pagesDDS NotesCINDY LIBANTINONo ratings yet
- Non Medical Prescribing Using The British National Formulary PDFDocument6 pagesNon Medical Prescribing Using The British National Formulary PDFRyan MwNo ratings yet
- Ferner 1993Document2 pagesFerner 1993Ashraf AlbhlaNo ratings yet
- Deficiency Letter of All Deferred Products in 91st Meeting of EEC Held On 13 07 2021Document62 pagesDeficiency Letter of All Deferred Products in 91st Meeting of EEC Held On 13 07 2021chemist7660No ratings yet
- Interaksi 03Document6 pagesInteraksi 03Nanda Asyura RizkyaniNo ratings yet
- ISR NG Chun Ruh2Document1 pageISR NG Chun Ruh2Yasmine SlhNo ratings yet
- L22-Animal Drug Residues in FoodDocument10 pagesL22-Animal Drug Residues in FoodCh LiuNo ratings yet
- GuideDocument128 pagesGuidebgtbingoNo ratings yet
- Pharmacology FinalDocument16 pagesPharmacology FinalDeepak MurthyNo ratings yet