Professional Documents
Culture Documents
2004-1
2004-1
Uploaded by
brightnewworld365Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2004-1
2004-1
Uploaded by
brightnewworld365Copyright:
Available Formats
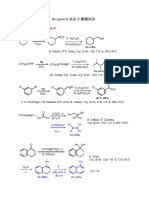
Fukuyama Group-Group Meeting Cumulative Examination 4/17/04
1.
Me O Me OH
N
NOCl hν
Me H OH Me H O
pyridine EtOH
H H H H
H H
H. Suginome et al., J. Org. Chem., 54, 5945 (1989)
2.
1) CHBr3, CH2Cl2
aq NaOH (MeCO)2, hν CO2H
phase-transfer cat., 45% 58%
Cl Cl C5H6
2) MeLi (2 eq), –78 to 0 °C 3) Br2, aq NaOH HO2C
Et2O-pentane; 1,4-dioxane, 90%
Michl, J. et al., J. Org. Chem. 53, 4593 (1988)
3.
3) MgX
Ph Ph 1) I(coll)2ClO4
medium toluene, rt OH
OMe
HO membered 86%
O O
CH2Cl2 ring
compound 4) Ca, NH3
–78 °C to rt, 77 % OH
–78 °C, 92%
2) LAH, THF
72%
Y. Kita et al., J. Org. Chem., 61, 7309 (1996)
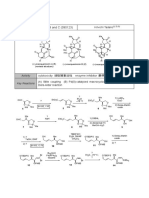
1) BH3•THF
4. 2) CH2=C(OMe)Me
OH PPTS
1) AcCl
CO2H ? ?
HO2C
2) EtOH (Compound) 3) NaOEt, EtOH (Compound)
71%
?
(Name)
1) ?
BF3•Et2O O O 2) ? O O
EtO2C I
Et2O 3) ?
100% (Conditions) K. Mori (Olean)
PhI(OAc)2
BzO O 1) ? BzO O I2
OH ?
2) ? hν (Compound)
(Conditions) CN (tungsten lamp)
O
11
S. Hatakeyama (Paeoniflorin)
天然物の全合成, p. 161 and pp. 167-168
5. In the late 1950s R. B. Woodward published his now classic synthesis of the alkaloid reserpine. Woodward
utilized the reaction of 1 (eq. 1) because 3 (eq. 2) was unreactive towards the desired epimerization.
H N H H N H
t-BuCO2H
N N (1)
MeO MeO
H O H O
H H
OMe OMe
O O
1 2
H N H H N H
t-BuCO2H
OAc OAc
N N (2)
MeO MeO
H H H H
MeO2C OMe MeO2C OMe
3 4
A. Provide a general mechanism for the equilibration of 1 to 2. 3-D drawings are not required.
B. Provide clear 3-D drawings of the strating materials and products (1-4). Use them to explain the observed
divergence in reactivity.
You might also like
- 2nd Second Quarter Exam Gen ChemDocument16 pages2nd Second Quarter Exam Gen Chemgodwin05092007No ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 2008-1Document1 page2008-1brightnewworld365No ratings yet
- Fukuyama Group - Group Meeting Problems 2001/08/22: N N N HDocument2,429 pagesFukuyama Group - Group Meeting Problems 2001/08/22: N N N HGia PhướcNo ratings yet
- 2018 PDFDocument32 pages2018 PDFDicky Tak Hin WongNo ratings yet
- 2008-2Document2 pages2008-2brightnewworld365No ratings yet
- 2008-3Document2 pages2008-3brightnewworld365No ratings yet
- Kaitocephalin-2 - USDocument2 pagesKaitocephalin-2 - USPercival GalahadNo ratings yet
- Cum 130420Document1 pageCum 130420Huỳnh ĐặngNo ratings yet
- Group Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)Document5 pagesGroup Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)dicky wongNo ratings yet
- Short Answer Questions: Chem223 Practice Exam (Pre Final)Document2 pagesShort Answer Questions: Chem223 Practice Exam (Pre Final)Jenny WangNo ratings yet
- Activity: O Me Me BR H OHDocument2 pagesActivity: O Me Me BR H OHPercival GalahadNo ratings yet
- Fukuyama Group - Group Meeting Problems 07/04/2017Document4 pagesFukuyama Group - Group Meeting Problems 07/04/2017Huỳnh ĐặngNo ratings yet
- Activity: O H OH OH OH OH O HDocument3 pagesActivity: O H OH OH OH OH O HPercival GalahadNo ratings yet
- Key 3Document4 pagesKey 3stgdk9gyf8No ratings yet
- Problem Session RemiDocument1 pageProblem Session RemiStudent BpharmaNo ratings yet
- Group Meeting Problems 2023/04/08: O Me Me Me OH Me Me Me O HODocument7 pagesGroup Meeting Problems 2023/04/08: O Me Me Me OH Me Me Me O HOroccenxuNo ratings yet
- The Ethylene Acetal A Was Also Prepared by An Alternative ApproachDocument45 pagesThe Ethylene Acetal A Was Also Prepared by An Alternative Approachbann tvNo ratings yet
- (+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityDocument3 pages(+) - Lasonolide A (120414-TKGP) K. Shishido: ActivityPercival GalahadNo ratings yet
- Chem 212 Condensation Reactions 3Document1 pageChem 212 Condensation Reactions 3kevinamyNo ratings yet
- Ingenol K. Tanino, I. Kuwajima: ActivityDocument2 pagesIngenol K. Tanino, I. Kuwajima: ActivityPercival GalahadNo ratings yet
- Aromatic Problems 2013Document4 pagesAromatic Problems 2013YocobSamandrewsNo ratings yet
- Organic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Document4 pagesOrganic Chemistry 2 Homework 2: Vo Lam Hoai Trung BTCEIU19009Trung VõNo ratings yet
- Synthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionDocument16 pagesSynthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionNguyễn Thái DươngNo ratings yet
- Group Meeting Problems 2021/11/06: Fac-Ir (Ppy) (0.2 Mol%) Dmso, RT 5W Blue Leds 67% O O O N O O O + N Ir N NDocument6 pagesGroup Meeting Problems 2021/11/06: Fac-Ir (Ppy) (0.2 Mol%) Dmso, RT 5W Blue Leds 67% O O O N O O O + N Ir N Ndicky wongNo ratings yet
- Laulimalide - USDocument4 pagesLaulimalide - USPercival GalahadNo ratings yet
- Pract Prob Carboxylic Acids AnsDocument3 pagesPract Prob Carboxylic Acids AnsVictor HernandezNo ratings yet
- cabroxylic acidsDocument9 pagescabroxylic acidssrinivasnarne78No ratings yet
- e1e4b300ffc5fbe8f9d2830d555e0a4fDocument8 pagese1e4b300ffc5fbe8f9d2830d555e0a4fveenayaksachinsharmaNo ratings yet
- Ejercicios QO-I-T1 2Document6 pagesEjercicios QO-I-T1 2dddddNo ratings yet
- 10.2 Grignard 反応と関連反応: Mg Et O 1) 2) H SO /H ODocument8 pages10.2 Grignard 反応と関連反応: Mg Et O 1) 2) H SO /H OHiman KumarNo ratings yet
- CHEM F311 Lecture 37 Mannich Reaction and Use of Specific Enol EquivalentsDocument6 pagesCHEM F311 Lecture 37 Mannich Reaction and Use of Specific Enol Equivalentsliving luxuriousNo ratings yet
- (+) - Macquarimicins A, B and C (090123) : Kin-Ichi TadanoDocument3 pages(+) - Macquarimicins A, B and C (090123) : Kin-Ichi TadanoPercival GalahadNo ratings yet
- Letitia Biweeklyreport 2Document5 pagesLetitia Biweeklyreport 2Anonymous hCWbXhgNo ratings yet
- Y2 B&SiDocument6 pagesY2 B&SiBin RenNo ratings yet
- Selectivity in Organic SynthesiDocument5 pagesSelectivity in Organic SynthesiChris LittleNo ratings yet
- Adv Retrosynthesis PDFDocument29 pagesAdv Retrosynthesis PDFericaNo ratings yet
- Jee Main Full Syllabus Test-3Document10 pagesJee Main Full Syllabus Test-3Haresh GNo ratings yet
- Pestalotiopsin A - USDocument2 pagesPestalotiopsin A - USPercival GalahadNo ratings yet
- Exercise 14 - Carbonyl Chemistry: Claisen, Aldol Type-And 1,4-AdditionsDocument2 pagesExercise 14 - Carbonyl Chemistry: Claisen, Aldol Type-And 1,4-AdditionsAllalannNo ratings yet
- Journal Pre-Proofs: Bioorganic & Medicinal ChemistryDocument18 pagesJournal Pre-Proofs: Bioorganic & Medicinal ChemistryWalid Ebid ElgammalNo ratings yet
- Ethyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et ODocument4 pagesEthyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et OPhạm Gia KhánhNo ratings yet
- (E) - 4-Chloro-3-Buten-2-One: CL R O R R R H CL O O Et CO EtDocument2 pages(E) - 4-Chloro-3-Buten-2-One: CL R O R R R H CL O O Et CO EtTaciturnoait NihilistaNo ratings yet
- (-) - Pinnaic Acid (TKGP 110129) H. Arimoto, D. Uemura: ActivityDocument2 pages(-) - Pinnaic Acid (TKGP 110129) H. Arimoto, D. Uemura: ActivityPercival GalahadNo ratings yet
- Aldehydes and Ketones MainsDocument8 pagesAldehydes and Ketones MainsShiva Ram Prasad PulagamNo ratings yet
- 3 PDocument3 pages3 PHồ Đức ViệtNo ratings yet
- Provera Znanja 1209Document5 pagesProvera Znanja 1209ShomiNo ratings yet
- Organic Practice Set 11 Chapters 8 10Document4 pagesOrganic Practice Set 11 Chapters 8 10Macedih K EricNo ratings yet
- PracticeTests Answers All Chem360Document109 pagesPracticeTests Answers All Chem360EthanNo ratings yet
- Tutorial 2Document3 pagesTutorial 2Marlinda Marcus LundangNo ratings yet
- Madindoline A-TSU - USDocument2 pagesMadindoline A-TSU - USPercival GalahadNo ratings yet
- Iron - Catalysis (Furstner) PDFDocument35 pagesIron - Catalysis (Furstner) PDFludoNo ratings yet
- Final Exam (Answr Key-Org 1 (24-6-2012)Document5 pagesFinal Exam (Answr Key-Org 1 (24-6-2012)Amira BeltagyNo ratings yet
- Organic Chem. IV. BCH 2210 Cat 1 and Ii INSTRUCTIONS: 1. Answer ALL Questions in Section A (40 Marks)Document13 pagesOrganic Chem. IV. BCH 2210 Cat 1 and Ii INSTRUCTIONS: 1. Answer ALL Questions in Section A (40 Marks)Brian MbuguaNo ratings yet
- Total Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachDocument2 pagesTotal Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachChem MistryNo ratings yet
- Group Meeting Problems 2021/10/02Document9 pagesGroup Meeting Problems 2021/10/02dicky wongNo ratings yet
- Catalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Document2 pagesCatalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Chem MistryNo ratings yet
- Chem Academy: Enolate ChemistryDocument13 pagesChem Academy: Enolate ChemistryHamit Rana100% (1)
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- Fukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundDocument1 pageFukuyama Group - Group Meeting Problems 1/17/2012: Bicyclic CompoundAnonymous rhUNqC1s0No ratings yet
- Imperial College LondonDocument1 pageImperial College LondonCalum GlynnNo ratings yet
- PHY 103 Lecture 4Document26 pagesPHY 103 Lecture 4oloruntishevictorNo ratings yet
- How To Weld TIGDocument14 pagesHow To Weld TIGMarcel PantanoNo ratings yet
- N Comms 8332Document7 pagesN Comms 8332Dương 10 Hoá TiêuNo ratings yet
- Astm A957 A957m 13Document6 pagesAstm A957 A957m 13Diego CanilNo ratings yet
- Chapter 1 Classes of Org. CpdsDocument100 pagesChapter 1 Classes of Org. CpdsezgmsayleakeNo ratings yet
- Textbook Dopants and Defects in Semiconductors Second Edition Matthew D Mccluskey Ebook All Chapter PDFDocument53 pagesTextbook Dopants and Defects in Semiconductors Second Edition Matthew D Mccluskey Ebook All Chapter PDFtony.kirkpatrick168100% (15)
- Emollient For Cosmetic TestingDocument9 pagesEmollient For Cosmetic TestingAGNEL ANTONY RAJNo ratings yet
- Astm D 2896 - TBNDocument9 pagesAstm D 2896 - TBNkapilyuvaan06No ratings yet
- Acid and Bases 2024 DR MnguniDocument24 pagesAcid and Bases 2024 DR MngunimolotopotsoNo ratings yet
- ChemistryDocument4 pagesChemistryjhonlyoddomingo50No ratings yet
- Gr11 PS (ENG) June 2022 Question PaperDocument14 pagesGr11 PS (ENG) June 2022 Question Paperitsthatgir. panasheNo ratings yet
- 12 - Clear Soda Titration Lab 2023Document4 pages12 - Clear Soda Titration Lab 2023Mathar BashirNo ratings yet
- General Organic and Biological Chemistry 6Th Edition Stoker Test Bank Full Chapter PDFDocument36 pagesGeneral Organic and Biological Chemistry 6Th Edition Stoker Test Bank Full Chapter PDFallison.young656100% (22)
- Zero Carbon Industry Transformative Technologies and Policies To Achieve Sustainable Prosperity Jeffrey Rissman All ChapterDocument67 pagesZero Carbon Industry Transformative Technologies and Policies To Achieve Sustainable Prosperity Jeffrey Rissman All Chaptermargaret.appel145100% (11)
- 03 0620 42 6RP Afp M24 13022024090457Document12 pages03 0620 42 6RP Afp M24 13022024090457nlightacademy23No ratings yet
- Using Cyanex 923 For Selective Extraction in A High Concentration Chloride Medium-Part II-LarssonDocument8 pagesUsing Cyanex 923 For Selective Extraction in A High Concentration Chloride Medium-Part II-LarssonDaiana NavarreteNo ratings yet
- Black B 133%Document7 pagesBlack B 133%DHRUVNo ratings yet
- AnaChem Lec TransesDocument32 pagesAnaChem Lec TranseschennielafleurNo ratings yet
- 2015 Dse Chem 2 1Document8 pages2015 Dse Chem 2 1Henry NgNo ratings yet
- Introduction To Chemical Processes Principles Analysis Synthesis 2Nd Edition Regina Murphy Download 2024 Full ChapterDocument47 pagesIntroduction To Chemical Processes Principles Analysis Synthesis 2Nd Edition Regina Murphy Download 2024 Full Chapterfaye.kauffman119100% (11)
- Epikure 3380 TDSDocument3 pagesEpikure 3380 TDSfatemeh.ahmadkhaniNo ratings yet
- Literature Review of Paper ChromatographyDocument5 pagesLiterature Review of Paper Chromatographydafobrrif100% (1)
- Lecture Planner - Organic Chemistry - Varun JEE Advanced 2024Document1 pageLecture Planner - Organic Chemistry - Varun JEE Advanced 2024tomarayush570No ratings yet
- Lecture 1Document33 pagesLecture 1kiwandaemmanuel21No ratings yet
- EU Substances Testing MethodsDocument18 pagesEU Substances Testing MethodsalpersakirmetinNo ratings yet
- Practical Work N.3 Chemical EquilibriumDocument6 pagesPractical Work N.3 Chemical Equilibriummissipssabouchala272No ratings yet
- Full Chapter Process Safety Calculations 2Nd Edition Renato Benintendi PDFDocument53 pagesFull Chapter Process Safety Calculations 2Nd Edition Renato Benintendi PDFjamie.cea614100% (7)
- Modern Chemistry Chapter 13 Homework 13-3Document7 pagesModern Chemistry Chapter 13 Homework 13-3afeudgbfr100% (1)
- Class 9TH MCQ Chemistry All ChaptersDocument5 pagesClass 9TH MCQ Chemistry All Chaptersdebasmitdas95No ratings yet