Professional Documents
Culture Documents
Thorgeirsson The New England journal of medici
Thorgeirsson The New England journal of medici
Uploaded by
Madhwesh CrCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thorgeirsson The New England journal of medici
Thorgeirsson The New England journal of medici
Uploaded by
Madhwesh CrCopyright:
Available Formats
The n e w e ng l a n d j o u r na l of m e dic i n e
Sall4 in “Stemness”-Driven Hepatocarcinogenesis
Jens U. Marquardt, M.D., and Snorri S. Thorgeirsson, M.D., Ph.D.
Hepatocellular carcinoma is the most common molecule (EpCAM), SALL4 could be an oncofetal
and deadly cause of primary liver cancer. Al- marker and an attractive therapeutic target in
though the incidence of hepatocellular carcinoma hepatocellular carcinoma with progenitor-cell
is highest in Asia and sub-Saharan Africa, the origin (Fig. 1). The authors also report an onco
steadily increasing incidence in traditionally fetal reactivation of SALL4 in 95 of 171 hepatitis
low-incidence regions such as northern Europe B–associated hepatocellular carcinoma specimens
and the United States is a considerable public (55.6%) at different stages. They validated this
health problem.1 Despite implementation of sur- finding in an independent group of specimens
veillance programs, the majority of patients still obtained from 228 patients in Hong Kong who
present with advanced stages of the disease, for had hepatocellular carcinoma as well as human
which curative options are limited. hepatoma cell lines. Most importantly, high
Currently, the standard-of-care treatment for SALL4 expression was significantly associated
advanced liver cancer is limited to the multiple with overall survival among patients in both co-
receptor tyrosine kinase inhibitor sorafenib.2 horts in the study (the Hong Kong and Singa-
Furthermore, development of new therapies is pore cohorts) and was an independent prognos-
often hampered by the molecular complexity of tic factor in univariate analyses. Overexpressing
hepatocellular carcinoma as well as associated tumors had molecular profiles that were similar
inflammatory and fibrotic liver disease. Results to those of fetal progenitor cells and shared ac-
of ongoing clinical trials are discouraging over- tivation of adverse gene sets with aggressive
all, and they highlight the urgent and unmet stemness-associated hepatocellular carcinoma;
need for innovative treatment approaches in pa- these findings provided support for the oncofe-
tients with advanced stages of hepatocellular tal role of SALL4. Also, RNA interference–medi-
carcinoma.3 ated silencing of SALL4 reduced tumorigenicity,
Cells with “stemness,” or stem-cell proper- increased apoptosis in human hepatoma cell
ties, are referred to as cancer stem cells or can- lines, and caused a reversal of the stemlike mo-
cer-initiating cells. The concept that these cells lecular profile of the cells. By using a specific
rest at the apex of the cancer hierarchy is an inhibitory 12-AA peptide against SALL4, the au-
evolving theme in cancer research.4 These cells thors convincingly showed that SALL4 affects
are by definition primarily responsible for initi- phosphatase and tensin homologue (PTEN) and
ation and propagation of tumors as well as re- phosphatidylinositol 3-kinase (PI3K)–AKT signal-
lapse after therapy, and they are therefore of ing through the interaction with the NuRD (nu-
major scientific interest. Several studies indicate cleosome remodeling and histone deacetylase
that hepatocellular carcinomas that harbor phe- [HDAC]) complex. These mechanistic findings
notypic features of stem cells and progenitor extend those of a recent study that independently
cells constitute a subclass of therapeutically provided evidence that SALL4 is a marker of stem-
challenging cancers that are associated with a cell–driven hepatocarcinogenesis and poor prog-
particularly poor prognosis.5 One of the stem- nosis.9 Finally, the authors elegantly showed a
ness genes associated with cancer is the human biologic activity of the peptide and thereby
homologue of the Drosophila spalt homeotic confirmed a potential therapeutic role for it in
gene, Sall4, which encodes a zinc-finger tran- SALL4-expressing tumors with stemlike fea-
scription factor that is essential for the mainte- tures and poor outcome.
nance of pluripotent and tissue stem cells.6 In Although these data are convincing, it is not
the liver, SALL4 is expressed at high levels in fe- known whether targeting of SALL4 alone would
tal-liver progenitor cells but not in adult hepato- have sufficient antitumor activity or whether in-
cytes, and it plays a critical role in hepatic cell– hibition of the associated transcriptomic pro-
lineage commitment.7 grams is required to prevent recurrent disease.
In this issue of the Journal, Yong and col- Another open question is whether responses to
leagues8 hypothesize that, like other stem-cell SALL4 inhibition in stemness-associated tumors
markers, such as the epithelial-cell adhesion vary according to the patient’s ethnic origin and
2316 n engl j med 368;24 nejm.org june 13, 2013
The New England Journal of Medicine
Downloaded from nejm.org at Boehringer-Ingelheim Pharma KG on September 15, 2014. For personal use only. No other uses without permission.
Copyright © 2013 Massachusetts Medical Society. All rights reserved.
editorials
Standard care
SALL4 Low
100 SALL4 low
SALL4 high
80
Survival (%)
60
40
20
0
0 20 40 60 80 100
Months
SALL4 High
PTEN AKT SALL4-based treatment
SALL4 peptide
PTEN AKT
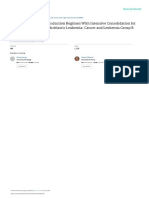
Figure 1. SALL4-Based Classification of Human Hepatocellular Carcinoma.
SALL4 expression divides hepatocellular carcinomas into clinically distinct subgroups. High SALL4 levels, which in-
dicate stemness-driven tumors with activation of the PTEN–PI3K–AKT pathway, predict aggressive tumor growth.
The graph shows the hypothetical rate of survival among patients with the two subgroups of hepatocellular carcino-
ma. Stratification of human hepatocellular carcinomas according to SALL4 expression appears to be a promising
method to select targeted therapies such as the SALL4 peptide in highly individualized treatment regimens.
the cause of the hepatocellular carcinoma (e.g., phase 2 trial of the c-MET inhibitor tivantinib;
hepatitis B virus, hepatitis C virus, and alcohol); this trial shows the necessity of mandatory biop-
this variability has been repeatedly observed in sies for molecular subclassification in patients
other targeted therapies.10 Also, the present data with advanced hepatocellular carcinomas.11 Fur-
do not resolve whether tumors that overexpress ther, Yong et al. elegantly show the benefit of
SALL4 are derived from reprogrammed hepato- innovative treatment strategies in hepatocellular
cytes or rare stem cells; the latter might have a carcinoma, including the targeting of genes
major effect on the regenerative capacity of the with stem-cell features such as SALL4. Clinical
liver and should be considered in a therapeutic translation of these important findings is ur-
context. gently needed to achieve individualized thera-
Clinically, the interaction of SALL4 with the pies and ultimately improve the poor outcome
HDAC-containing NuRD complex underlines the in patients with hepatocellular carcinoma.
promising results of an ongoing phase 2 trial of Disclosure forms provided by the authors are available with
the HDAC inhibitor resminostat (ClinicalTrials the full text of this article at NEJM.org.
.gov number, NCT00943449) and may warrant From the Laboratory of Experimental Carcinogenesis, Center
further subgroup analyses in this trial. The sys- for Cancer Research, National Cancer Institute, National Insti-
tutes of Health, Bethesda, MD (J.U.M., S.S.T.); and the Depart-
tematically performed study by Yong and col- ment of Medicine I, Johannes Gutenberg University, Mainz,
leagues also provides support for findings in a Germany (J.U.M.).
n engl j med 368;24 nejm.org june 13, 2013 2317
The New England Journal of Medicine
Downloaded from nejm.org at Boehringer-Ingelheim Pharma KG on September 15, 2014. For personal use only. No other uses without permission.
Copyright © 2013 Massachusetts Medical Society. All rights reserved.
editorials
1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lan- fate decision in fetal hepatic stem/progenitor cells. Gastroenter-
cet 2012;379:1245-55. ology 2009;136:1000-11.
2. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced 8. Yong KJ, Gao C, Lim JSJ, et al. Oncofetal gene SALL4 in aggres-
hepatocellular carcinoma. N Engl J Med 2008;359:378-90. sive hepatocellular carcinoma. N Engl J Med 2013;368:2266-76.
3. Forner A, Llovet JM, Bruix J. Sunitinib and the benefits of a 9. Oikawa T, Kamiya A, Zeniya M, et al. Sal-like protein 4
negative study. Lancet Oncol 2009;10:743-4. (SALL4), a stem cell biomarker in liver cancers. Hepatology
4. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl 2013;57:1469-83.
J Med 2006;355:1253-61. 10. Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of
5. Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype sorafenib in patients with advanced hepatocellular carcinoma:
of human hepatocellular carcinoma derived from hepatic pro- subanalyses of a phase III trial. J Hepatol 2012;57:821-9.
genitor cells. Nat Med 2006;12:410-6. 11. Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-
6. Yang J, Chai L, Gao C, et al. SALL4 is a key regulator of sur- line treatment of advanced hepatocellular carcinoma: a random-
vival and apoptosis in human leukemic cells. Blood 2008;112:805- ised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63.
13. [Erratum, Blood 2009;114:3131.] DOI: 10.1056/NEJMe1303026
7. Oikawa T, Kamiya A, Kakinuma S, et al. Sall4 regulates cell Copyright © 2013 Massachusetts Medical Society.
2318 n engl j med 368;24 nejm.org june 13, 2013
The New England Journal of Medicine
Downloaded from nejm.org at Boehringer-Ingelheim Pharma KG on September 15, 2014. For personal use only. No other uses without permission.
Copyright © 2013 Massachusetts Medical Society. All rights reserved.
You might also like
- Tenen 2013 NEJM SALL4 liverDocument11 pagesTenen 2013 NEJM SALL4 liverMadhwesh CrNo ratings yet
- Zhang Cancer Letter 2015Document8 pagesZhang Cancer Letter 2015Madhwesh CrNo ratings yet
- Chai Blood 2013Document9 pagesChai Blood 2013Madhwesh CrNo ratings yet
- AJPCR ArticleDocument4 pagesAJPCR ArticlepreethiNo ratings yet
- Clinical Implications of Our Advancing Knowledge of Colorectal Cancer Genetics: Inherited Syndromes, Prognosis, Prevention, Screening and TherapeuticsDocument31 pagesClinical Implications of Our Advancing Knowledge of Colorectal Cancer Genetics: Inherited Syndromes, Prognosis, Prevention, Screening and TherapeuticsprivedNo ratings yet
- 397 FullDocument6 pages397 FullNedhal Mahmoud KaleefahNo ratings yet
- fajas laia ref cdk4Document16 pagesfajas laia ref cdk4JaimeD.LópezAlcaláNo ratings yet
- G6PD and Cancer PathwaysDocument26 pagesG6PD and Cancer PathwaysSurabhiNo ratings yet
- Oncotarget-Poco Atf4 ResistenciaDocument14 pagesOncotarget-Poco Atf4 ResistenciaJaimeD.LópezAlcaláNo ratings yet
- Biomarker CA OvariumDocument7 pagesBiomarker CA OvariumArya Maulana NugrohoNo ratings yet
- Sequence, Treat, Repeat: Addressing Resistance in EGFR-Mutant NSCLCDocument3 pagesSequence, Treat, Repeat: Addressing Resistance in EGFR-Mutant NSCLCDan DiaconeasaNo ratings yet
- In Vitro and in VivoDocument12 pagesIn Vitro and in VivoLourdesNo ratings yet
- Fol Firin OxDocument11 pagesFol Firin OxGuilherme SalgadoNo ratings yet
- Cancer Discovery 2014 Lock 466 79Document15 pagesCancer Discovery 2014 Lock 466 79Daniel Juarez SerranoNo ratings yet
- JournalDocument7 pagesJournalYuanita Permata GunawanNo ratings yet
- Cell Stress ScopusDocument3 pagesCell Stress ScopusAmged GamalNo ratings yet
- Cancer Research Papers FreeDocument8 pagesCancer Research Papers Freeafnhdqfvufitoa100% (1)
- Referensi PDB ID For HER-2 (3PP0)Document12 pagesReferensi PDB ID For HER-2 (3PP0)eti apriyantiNo ratings yet
- 2020 Schcolnik Et AlDocument11 pages2020 Schcolnik Et AlArturo RojoNo ratings yet
- TargetingDocument1 pageTargetingrcandelaNo ratings yet
- مقالههههDocument34 pagesمقالههههyosof esfandiariNo ratings yet
- Gonzalez-Sanchez Et Al Cancers 2021Document24 pagesGonzalez-Sanchez Et Al Cancers 2021mafe.z.solarte87No ratings yet
- Mustachio 2020Document14 pagesMustachio 2020Alfiah NoorNo ratings yet
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines For CYP3A5 Genotype and Tacrolimus DosingDocument6 pagesClinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines For CYP3A5 Genotype and Tacrolimus DosingLekshmy SrinivasNo ratings yet
- Bmi s16553Document8 pagesBmi s16553Cassandra VérasNo ratings yet
- FileDocument8 pagesFileananda febriani auliaNo ratings yet
- tmp3AAA TMPDocument10 pagestmp3AAA TMPFrontiersNo ratings yet
- Final Synthesis PaperDocument19 pagesFinal Synthesis Paperapi-447485924No ratings yet
- Hippo CancerDocument7 pagesHippo CancerMaria Elena MaldonadoNo ratings yet
- Ajcr0008 2436Document21 pagesAjcr0008 2436louisehip UFCNo ratings yet
- 2012cancprevres 5 351 4 Metformin Cscs - SemimDocument5 pages2012cancprevres 5 351 4 Metformin Cscs - SemimYolita Satya Gitya UtamiNo ratings yet
- This Is Your Thyroid On Drugs Targetable Mutations An 2023 Surgical PatholoDocument17 pagesThis Is Your Thyroid On Drugs Targetable Mutations An 2023 Surgical PatholorubenmacaNo ratings yet
- The Silencing of Long Non-Coding RNA ANRIL Suppresses Invasion, and Promotes Apoptosis of Retinoblastoma Cells Through The ATM-E2F1 Signaling PathwayDocument29 pagesThe Silencing of Long Non-Coding RNA ANRIL Suppresses Invasion, and Promotes Apoptosis of Retinoblastoma Cells Through The ATM-E2F1 Signaling PathwayJosué Cristhian Del Valle HornaNo ratings yet
- AlsnlasnfDocument13 pagesAlsnlasnfDina A. ŠabićNo ratings yet
- Resistant Breast Cancer Aberrantly Activated by PI3K/AKT Hedgehog Signaling Is A Novel Therapeutic Target in TamoxifenDocument39 pagesResistant Breast Cancer Aberrantly Activated by PI3K/AKT Hedgehog Signaling Is A Novel Therapeutic Target in TamoxifentriwiyonoNo ratings yet
- Regimen Calgb 8811Document14 pagesRegimen Calgb 8811ricardo_cheNo ratings yet
- The Interplay Between - TspaceDocument35 pagesThe Interplay Between - TspaceArooNo ratings yet
- Cancer Testis1234Document10 pagesCancer Testis1234Defi MarizalNo ratings yet
- A Comparison of Physicochemical Property Profiles of Marketed Oral Drugs and Orally Bioavailable Anti-Cancer Protein Kinase Inhibitors in Clinical DevelopmentDocument16 pagesA Comparison of Physicochemical Property Profiles of Marketed Oral Drugs and Orally Bioavailable Anti-Cancer Protein Kinase Inhibitors in Clinical DevelopmentArturo T. Sánchez-MoraNo ratings yet
- Cancers 13 04088 v3Document18 pagesCancers 13 04088 v3xjwwm99v6nNo ratings yet
- The Breast Journal - 2015 - Chin - CYP2D6 Genetic Polymorphisms and Phenotypes in Different Ethnicities of Malaysian BreastDocument9 pagesThe Breast Journal - 2015 - Chin - CYP2D6 Genetic Polymorphisms and Phenotypes in Different Ethnicities of Malaysian BreastDR. NORHASHIMAH BT ABU SEMAN (NIH)No ratings yet
- Exciting New Targets in Lung Cancer Therapy: ALK, IGF-1R, HDAC, and HHDocument9 pagesExciting New Targets in Lung Cancer Therapy: ALK, IGF-1R, HDAC, and HHJaneFinnNo ratings yet
- SasdsadsaDocument11 pagesSasdsadsaJorge MariaNo ratings yet
- Biomarkers and Algorithms For Diagnosis of Ovarian CancerDocument9 pagesBiomarkers and Algorithms For Diagnosis of Ovarian CancerGeorgiana Daniela DragomirNo ratings yet
- New Treatment Options For Metastatic Thyroid CancerDocument6 pagesNew Treatment Options For Metastatic Thyroid CancermarcelinaNo ratings yet
- Antiproliferative Effects and Mechanisms of Liver X Receptor Ligands in Pancreatic Ductal Adenocarcinoma CellsDocument11 pagesAntiproliferative Effects and Mechanisms of Liver X Receptor Ligands in Pancreatic Ductal Adenocarcinoma CellsscribdenesimoNo ratings yet
- Liao 2014Document13 pagesLiao 2014Jenivia LulileloNo ratings yet
- KAsner 2Document20 pagesKAsner 2sabarinaramNo ratings yet
- Clinical Chemistry Question and Answer: Answered By: Edward Stelow, M.D., Pathology ResidentDocument1 pageClinical Chemistry Question and Answer: Answered By: Edward Stelow, M.D., Pathology Residentsuhaila bakhtanNo ratings yet
- Jurnal 4Document9 pagesJurnal 4YTNo ratings yet
- 2011 04 Clin Cancer Res - AfzalDocument9 pages2011 04 Clin Cancer Res - AfzalEspen Jimenez SolemNo ratings yet
- Stampferetal 2013 SpringerDocument40 pagesStampferetal 2013 SpringerPilar AufrastoNo ratings yet
- Anti CancerDocument12 pagesAnti CancerAlarofatusnainiNo ratings yet
- Acute Promyelocytic Leukemia (APL) : A Review of The LiteratureDocument12 pagesAcute Promyelocytic Leukemia (APL) : A Review of The LiteratureStephan Arvid Skog MarambioNo ratings yet
- PIIS1535610819302971Document11 pagesPIIS1535610819302971saraanpkNo ratings yet
- Biomarkers in Breast Cancer 2024: An Updated Consensus Statement by The Spanish Society of Medical Oncology and The Spanish Society of PathologyDocument17 pagesBiomarkers in Breast Cancer 2024: An Updated Consensus Statement by The Spanish Society of Medical Oncology and The Spanish Society of PathologyellyanaperwitasariNo ratings yet
- Stamelou 2021Document20 pagesStamelou 2021Alex DimanceaNo ratings yet
- Cancer UpdateDocument19 pagesCancer UpdatePetra JobovaNo ratings yet
- Targeted Therapies for Lung CancerFrom EverandTargeted Therapies for Lung CancerRavi SalgiaNo ratings yet
- Genetic Interactions Between Specific Chromosome Copy Number Alterations Dictate Complex Aneuploidy PatternsDocument14 pagesGenetic Interactions Between Specific Chromosome Copy Number Alterations Dictate Complex Aneuploidy PatternsMadhwesh CrNo ratings yet
- Structure of The Helicase Core of Werner Helicase, A Key Target in Microsatellite Instability CancersDocument13 pagesStructure of The Helicase Core of Werner Helicase, A Key Target in Microsatellite Instability CancersMadhwesh CrNo ratings yet
- Interrogation of Cancer Gene Dependencies Reveals Novel Paralog Interactions of Autosome and Sex Chromosome Encoded GenesDocument40 pagesInterrogation of Cancer Gene Dependencies Reveals Novel Paralog Interactions of Autosome and Sex Chromosome Encoded GenesMadhwesh CrNo ratings yet
- STAG1 Vulnerabilities For Exploiting Cohesin Synthetic Lethality in STAG2-deficient CancersDocument14 pagesSTAG1 Vulnerabilities For Exploiting Cohesin Synthetic Lethality in STAG2-deficient CancersMadhwesh CrNo ratings yet
- Prioritizing Synthetic Lethal Targets With Functional Genomics.Document1 pagePrioritizing Synthetic Lethal Targets With Functional Genomics.Madhwesh CrNo ratings yet
- Anime: The Future of Magick and OccultDocument12 pagesAnime: The Future of Magick and OccultPinball85No ratings yet
- Far Trek Continue VoyagesDocument148 pagesFar Trek Continue Voyageswintermute57100% (2)
- M8 Sensor UGuide 96-00001 REV H 073117Document50 pagesM8 Sensor UGuide 96-00001 REV H 073117Ashish KundapurNo ratings yet
- Assignment 2 Inorganic ChemistryDocument2 pagesAssignment 2 Inorganic Chemistryinam ullahNo ratings yet
- Warrant: Catherine Lynn JarveyDocument4 pagesWarrant: Catherine Lynn JarveyLeigh EganNo ratings yet
- Before Women Had WingsDocument5 pagesBefore Women Had WingsGianna RamirezNo ratings yet
- Industry Alloted Property NoidaDocument193 pagesIndustry Alloted Property Noidaavinashtyagi0% (1)
- EE 413-Engg ElectromagneticsDocument2 pagesEE 413-Engg ElectromagneticsVan GonzalesNo ratings yet
- Anh 7 Kim Hien Unit 7Document8 pagesAnh 7 Kim Hien Unit 7Hiếu Nguyễn MinhNo ratings yet
- Hvac Performance Analysis For A Conference Room: Visvesvaraya Technological UniversityDocument27 pagesHvac Performance Analysis For A Conference Room: Visvesvaraya Technological UniversityRohanRayNo ratings yet
- WiproDocument21 pagesWiproAnu TanjaNo ratings yet
- Bsm6000 Ug Part II-hDocument0 pagesBsm6000 Ug Part II-hMiguel Ángel Escobar FloresNo ratings yet
- 2020 79th PNWIMC Annual Conference AnnouncementDocument2 pages2020 79th PNWIMC Annual Conference AnnouncementnwberryfoundationNo ratings yet
- PPC Researched PointersDocument4 pagesPPC Researched PointershitmonNo ratings yet
- Sample Question Paper - 3: Class - XDocument17 pagesSample Question Paper - 3: Class - XRehan AlamNo ratings yet
- Analysis and Optimization of Spiral Plate Heat Exchanger Using Computational Fluid DynamicsDocument10 pagesAnalysis and Optimization of Spiral Plate Heat Exchanger Using Computational Fluid DynamicsIJRASETPublicationsNo ratings yet
- From Georges Sorel - Essays in Socialism and PhilosophyDocument411 pagesFrom Georges Sorel - Essays in Socialism and PhilosophyHugãoNo ratings yet
- Mens TshirtDocument15 pagesMens TshirtTrung Hieu NguyenNo ratings yet
- A Crisis Intervention Model For Child Protective S PDFDocument22 pagesA Crisis Intervention Model For Child Protective S PDFKali HartvigsenNo ratings yet
- 2018 Sigma Natural Ebook OPDocument55 pages2018 Sigma Natural Ebook OPRenan Norbiate de Melo100% (1)
- Extract of All Sales Vouchers 1-Apr-2015 To 31-Dec-2015Document8 pagesExtract of All Sales Vouchers 1-Apr-2015 To 31-Dec-2015taseerNo ratings yet
- The Polarization of Light: Experiment 3Document4 pagesThe Polarization of Light: Experiment 3Mujeeb UllahNo ratings yet
- Fleming's Left Hand Rule (ForDocument8 pagesFleming's Left Hand Rule (Forb_geyl4286No ratings yet
- Unit 1 Chapter 3: Quadratic FunctionsDocument18 pagesUnit 1 Chapter 3: Quadratic FunctionsZlata OsypovaNo ratings yet
- Extra NumericalsDocument2 pagesExtra NumericalsDeep KambleNo ratings yet
- AccurioLabel 400 - Basic - Demo - Script - v1.0 - EDocument33 pagesAccurioLabel 400 - Basic - Demo - Script - v1.0 - EDiego LanderosNo ratings yet
- Materi2012.1pre Print - Part1Document174 pagesMateri2012.1pre Print - Part1Hari TulusNo ratings yet
- Work Measurements and Methods: Uses of Setting Work StandardsDocument3 pagesWork Measurements and Methods: Uses of Setting Work StandardsSonaNo ratings yet
- Essential University Physics 3rd Edition by Wolfson ISBN Test BankDocument23 pagesEssential University Physics 3rd Edition by Wolfson ISBN Test Bankandrea100% (32)
- Wire Nails Manufacturing Business. How To Start Nail Factory. Profitable Small Business Ideas in India-887813Document63 pagesWire Nails Manufacturing Business. How To Start Nail Factory. Profitable Small Business Ideas in India-887813MuazNo ratings yet