Professional Documents
Culture Documents
Termodinámica 2
Termodinámica 2
Uploaded by
Jose Adan Alfonso TorresCopyright:
Available Formats
You might also like
- Preview FileDocument2 pagesPreview FilemalvinalaiNo ratings yet
- מטלת הגשה 26.2Document2 pagesמטלת הגשה 26.2Maayan HarpazNo ratings yet
- Ejercicios Entrega Tema 5Document4 pagesEjercicios Entrega Tema 5Andrea Garcia EstellesNo ratings yet
- Ejercicio Hiperestatico EnrejadoDocument2 pagesEjercicio Hiperestatico EnrejadoScarlett ParedesNo ratings yet
- Cambridge Mechanics Practices 1Document2 pagesCambridge Mechanics Practices 1Some GuyNo ratings yet
- Tarea 3, TareaDocument4 pagesTarea 3, Tareahy9v7z46mgNo ratings yet
- FINALSDocument2 pagesFINALSjrcruzpogi0242424No ratings yet
- Cambridge Mechanics Practices 3Document3 pagesCambridge Mechanics Practices 3Some GuyNo ratings yet
- MatekDocument1 pageMatekGyevnar GergoNo ratings yet
- Bono FísicaDocument2 pagesBono FísicaSara SolarteNo ratings yet
- Modelo LogísticoDocument1 pageModelo LogísticoVania FarreraNo ratings yet
- Tarca: Ao JA Ao EA (A (A yDocument2 pagesTarca: Ao JA Ao EA (A (A yRafael GuzmánNo ratings yet
- IE Actividad 3Document2 pagesIE Actividad 3alejadraNo ratings yet
- Sheet 2Document4 pagesSheet 2fatima.alansari55No ratings yet
- Untitled NotebookDocument2 pagesUntitled NotebookPhi ThườngNo ratings yet
- Ejercicio ExtraDocument2 pagesEjercicio ExtraMARIA FERNANDA GONZALES MONTENEGRONo ratings yet
- Ejercicios ManómetrosDocument2 pagesEjercicios ManómetrosAna NavarroNo ratings yet
- Exam 2 Part ADocument8 pagesExam 2 Part Aleodiaz215No ratings yet
- 物理hwDocument1 page物理hwljiezhi90No ratings yet
- 個經實習上課紀錄 2Document1 page個經實習上課紀錄 2rcccc2004No ratings yet
- Constan: ArmassDocument7 pagesConstan: ArmassSalim RihaniNo ratings yet
- Bloc de Notas Sin TítuloDocument1 pageBloc de Notas Sin TítuloDaniela ArdilaNo ratings yet
- Usxrstlook: STRL 100Document2 pagesUsxrstlook: STRL 100nycucumber.ee11No ratings yet
- ENCI - Assignment 5Document7 pagesENCI - Assignment 5coolvic909No ratings yet
- Wuolah Free Ejercicios T7 Gulag FreeDocument4 pagesWuolah Free Ejercicios T7 Gulag FreeuserwuolahNo ratings yet
- Cycle and Engine Analysis of An Ideal Rankine Cycle 3Document11 pagesCycle and Engine Analysis of An Ideal Rankine Cycle 3Sean GoNo ratings yet
- Deber2 AdrianSalvador 320811Document3 pagesDeber2 AdrianSalvador 320811asalvador16107No ratings yet
- Constraint MotionDocument5 pagesConstraint MotionnithinjothimuruganNo ratings yet
- Dimensi: Segitiga Ta BQ CP Éav3 Ia TaDocument10 pagesDimensi: Segitiga Ta BQ CP Éav3 Ia TaDavina AlmaNo ratings yet
- Mecánica de materiales ll 3Document3 pagesMecánica de materiales ll 3geezyxavieralNo ratings yet
- Janjang ArithmetikDocument15 pagesJanjang ArithmetikHaninii Suhaila HKNo ratings yet
- Talleres FísicaDocument1 pageTalleres FísicaLaura De BrigardNo ratings yet
- VBGH Gépész Gyak 3Document10 pagesVBGH Gépész Gyak 3Levente NagyNo ratings yet
- Heimadæmi 2 Burðarþol Eva Dís Og DíanaDocument5 pagesHeimadæmi 2 Burðarþol Eva Dís Og DíanadianamgenedyNo ratings yet
- Electrostatics PDFDocument5 pagesElectrostatics PDFshashikantNo ratings yet
- Cuestión 7 Entregar T 5.1Document1 pageCuestión 7 Entregar T 5.1al414631No ratings yet
- Pigeon TheoremDocument1 pagePigeon Theoremthinhandre12No ratings yet
- Tarea 4Document7 pagesTarea 4Santiago MartinezNo ratings yet
- Lms TurunanDocument9 pagesLms TurunanNovrizal Nur ANo ratings yet
- Excercise - Physics 2Document4 pagesExcercise - Physics 2Peter Bryant SoefianNo ratings yet
- Dynamics HW3Document1 pageDynamics HW3aa8846No ratings yet
- 1715015249801249Document119 pages1715015249801249valya surmeyNo ratings yet
- Circular Motion RevisionDocument2 pagesCircular Motion RevisionSuchit GuptaNo ratings yet
- Fyzika PraxeDocument1 pageFyzika PraxeJachymNo ratings yet
- OrezovDocument1 pageOrezovJachymNo ratings yet
- Bloc de Notas Sin TítuloDocument1 pageBloc de Notas Sin Títulomaria paula uribe coyNo ratings yet
- Tecnologia 2 Bat 2Document1 pageTecnologia 2 Bat 2Sofia Benet IranzoNo ratings yet
- Capacitor PDFDocument3 pagesCapacitor PDFshashikantNo ratings yet
- Proyecto Resis 2Document2 pagesProyecto Resis 2Daniel RamirezNo ratings yet
- Untitled (Draft)Document1 pageUntitled (Draft)SzuanlingNo ratings yet
- Lac-Most (Ca/A (Yrha: Se+S Exrha-3'UbvDocument4 pagesLac-Most (Ca/A (Yrha: Se+S Exrha-3'UbvriicardlopezNo ratings yet
- Img 20201030 0007Document1 pageImg 20201030 0007Michał KNo ratings yet
- Pure Maths Notes 3Document1 pagePure Maths Notes 3nevasimsek123No ratings yet
- Clàudia Pubill - Ejercicio Individual - Articuladas IsostáticasDocument1 pageClàudia Pubill - Ejercicio Individual - Articuladas IsostáticasClàudia Lídia Pubill QuintillàNo ratings yet
- 1 1 1 18Document3 pages1 1 1 18nycucumber.ee11No ratings yet
- 07 March Class Notes S3 Express E-MathsDocument5 pages07 March Class Notes S3 Express E-Mathsreinhard.rasimanNo ratings yet
- Ejercicio en Clase EstaticaDocument1 pageEjercicio en Clase EstaticaEmmanuel RodriguezNo ratings yet
- Tuto C3Document6 pagesTuto C3anitanazira30No ratings yet
- Negativex: JbedlDocument4 pagesNegativex: Jbedl陳泓睿No ratings yet
- The Rough Guide to Beijing (Travel Guide eBook)From EverandThe Rough Guide to Beijing (Travel Guide eBook)Rating: 2 out of 5 stars2/5 (1)
Termodinámica 2
Termodinámica 2
Uploaded by
Jose Adan Alfonso TorresCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Termodinámica 2
Termodinámica 2
Uploaded by
Jose Adan Alfonso TorresCopyright:
Available Formats
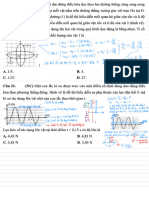
III
& as monoatómico -
(p =
2, 5Ri(v =
15R
a) ab e presión te U
n(pa+ a ()
S
- =
= =
n 2 5R A +...
,
. .
nRT () C R
Pa(Vpa
pV = en + =
.
Cab
*
=
2 25
,
x10 J
↓ w
Au
=
pAV
= Q
=
w
0 ,
90x055
*
Du =
1 , 35x10 J
S b)V
&- nat
Volumen contante
>(Pc-
bc a -
=
...
()
nRT
pV = ... en (2)
a = -
2
,
40x1055
↳ w = 0
Au = a -
w
bu = 2
,
4x105
ca e
presion y
volumen vanables a W
=- par = Area del
diagram
( )b)h (3x105 mx000
b) gv = 0 w = -
= +
-
0, 2)
*
A =
0 , 30X10 J
*
W =
0 , 30x10 J W = -
6x10"J
Au =
19Vabl-1AUbc =
1, 05x1055
↳
c) Wi =
Wab + Nba + Waa = 2, 7x1055 al final del ciclo Aut = 0
Au =
a -
w + a = Au + w = 030x1055
e 030xo %
W
,=
= = =
0
2, 70x105
You might also like
- Preview FileDocument2 pagesPreview FilemalvinalaiNo ratings yet
- מטלת הגשה 26.2Document2 pagesמטלת הגשה 26.2Maayan HarpazNo ratings yet
- Ejercicios Entrega Tema 5Document4 pagesEjercicios Entrega Tema 5Andrea Garcia EstellesNo ratings yet
- Ejercicio Hiperestatico EnrejadoDocument2 pagesEjercicio Hiperestatico EnrejadoScarlett ParedesNo ratings yet
- Cambridge Mechanics Practices 1Document2 pagesCambridge Mechanics Practices 1Some GuyNo ratings yet
- Tarea 3, TareaDocument4 pagesTarea 3, Tareahy9v7z46mgNo ratings yet
- FINALSDocument2 pagesFINALSjrcruzpogi0242424No ratings yet
- Cambridge Mechanics Practices 3Document3 pagesCambridge Mechanics Practices 3Some GuyNo ratings yet
- MatekDocument1 pageMatekGyevnar GergoNo ratings yet
- Bono FísicaDocument2 pagesBono FísicaSara SolarteNo ratings yet
- Modelo LogísticoDocument1 pageModelo LogísticoVania FarreraNo ratings yet
- Tarca: Ao JA Ao EA (A (A yDocument2 pagesTarca: Ao JA Ao EA (A (A yRafael GuzmánNo ratings yet
- IE Actividad 3Document2 pagesIE Actividad 3alejadraNo ratings yet
- Sheet 2Document4 pagesSheet 2fatima.alansari55No ratings yet
- Untitled NotebookDocument2 pagesUntitled NotebookPhi ThườngNo ratings yet
- Ejercicio ExtraDocument2 pagesEjercicio ExtraMARIA FERNANDA GONZALES MONTENEGRONo ratings yet
- Ejercicios ManómetrosDocument2 pagesEjercicios ManómetrosAna NavarroNo ratings yet
- Exam 2 Part ADocument8 pagesExam 2 Part Aleodiaz215No ratings yet
- 物理hwDocument1 page物理hwljiezhi90No ratings yet
- 個經實習上課紀錄 2Document1 page個經實習上課紀錄 2rcccc2004No ratings yet
- Constan: ArmassDocument7 pagesConstan: ArmassSalim RihaniNo ratings yet
- Bloc de Notas Sin TítuloDocument1 pageBloc de Notas Sin TítuloDaniela ArdilaNo ratings yet
- Usxrstlook: STRL 100Document2 pagesUsxrstlook: STRL 100nycucumber.ee11No ratings yet
- ENCI - Assignment 5Document7 pagesENCI - Assignment 5coolvic909No ratings yet
- Wuolah Free Ejercicios T7 Gulag FreeDocument4 pagesWuolah Free Ejercicios T7 Gulag FreeuserwuolahNo ratings yet
- Cycle and Engine Analysis of An Ideal Rankine Cycle 3Document11 pagesCycle and Engine Analysis of An Ideal Rankine Cycle 3Sean GoNo ratings yet
- Deber2 AdrianSalvador 320811Document3 pagesDeber2 AdrianSalvador 320811asalvador16107No ratings yet
- Constraint MotionDocument5 pagesConstraint MotionnithinjothimuruganNo ratings yet
- Dimensi: Segitiga Ta BQ CP Éav3 Ia TaDocument10 pagesDimensi: Segitiga Ta BQ CP Éav3 Ia TaDavina AlmaNo ratings yet
- Mecánica de materiales ll 3Document3 pagesMecánica de materiales ll 3geezyxavieralNo ratings yet
- Janjang ArithmetikDocument15 pagesJanjang ArithmetikHaninii Suhaila HKNo ratings yet
- Talleres FísicaDocument1 pageTalleres FísicaLaura De BrigardNo ratings yet
- VBGH Gépész Gyak 3Document10 pagesVBGH Gépész Gyak 3Levente NagyNo ratings yet
- Heimadæmi 2 Burðarþol Eva Dís Og DíanaDocument5 pagesHeimadæmi 2 Burðarþol Eva Dís Og DíanadianamgenedyNo ratings yet
- Electrostatics PDFDocument5 pagesElectrostatics PDFshashikantNo ratings yet
- Cuestión 7 Entregar T 5.1Document1 pageCuestión 7 Entregar T 5.1al414631No ratings yet
- Pigeon TheoremDocument1 pagePigeon Theoremthinhandre12No ratings yet
- Tarea 4Document7 pagesTarea 4Santiago MartinezNo ratings yet
- Lms TurunanDocument9 pagesLms TurunanNovrizal Nur ANo ratings yet
- Excercise - Physics 2Document4 pagesExcercise - Physics 2Peter Bryant SoefianNo ratings yet
- Dynamics HW3Document1 pageDynamics HW3aa8846No ratings yet
- 1715015249801249Document119 pages1715015249801249valya surmeyNo ratings yet
- Circular Motion RevisionDocument2 pagesCircular Motion RevisionSuchit GuptaNo ratings yet
- Fyzika PraxeDocument1 pageFyzika PraxeJachymNo ratings yet
- OrezovDocument1 pageOrezovJachymNo ratings yet
- Bloc de Notas Sin TítuloDocument1 pageBloc de Notas Sin Títulomaria paula uribe coyNo ratings yet
- Tecnologia 2 Bat 2Document1 pageTecnologia 2 Bat 2Sofia Benet IranzoNo ratings yet
- Capacitor PDFDocument3 pagesCapacitor PDFshashikantNo ratings yet
- Proyecto Resis 2Document2 pagesProyecto Resis 2Daniel RamirezNo ratings yet
- Untitled (Draft)Document1 pageUntitled (Draft)SzuanlingNo ratings yet
- Lac-Most (Ca/A (Yrha: Se+S Exrha-3'UbvDocument4 pagesLac-Most (Ca/A (Yrha: Se+S Exrha-3'UbvriicardlopezNo ratings yet
- Img 20201030 0007Document1 pageImg 20201030 0007Michał KNo ratings yet
- Pure Maths Notes 3Document1 pagePure Maths Notes 3nevasimsek123No ratings yet
- Clàudia Pubill - Ejercicio Individual - Articuladas IsostáticasDocument1 pageClàudia Pubill - Ejercicio Individual - Articuladas IsostáticasClàudia Lídia Pubill QuintillàNo ratings yet
- 1 1 1 18Document3 pages1 1 1 18nycucumber.ee11No ratings yet
- 07 March Class Notes S3 Express E-MathsDocument5 pages07 March Class Notes S3 Express E-Mathsreinhard.rasimanNo ratings yet
- Ejercicio en Clase EstaticaDocument1 pageEjercicio en Clase EstaticaEmmanuel RodriguezNo ratings yet
- Tuto C3Document6 pagesTuto C3anitanazira30No ratings yet
- Negativex: JbedlDocument4 pagesNegativex: Jbedl陳泓睿No ratings yet
- The Rough Guide to Beijing (Travel Guide eBook)From EverandThe Rough Guide to Beijing (Travel Guide eBook)Rating: 2 out of 5 stars2/5 (1)