Professional Documents
Culture Documents
Chromatography
Chromatography
Uploaded by
Yerram Raju BeharaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chromatography
Chromatography
Uploaded by
Yerram Raju BeharaCopyright:
Available Formats
1
S.Y B. Sc. AC 202 Unit III CHROMATOGRAPHY
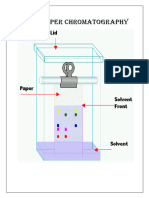
INTRODUCTION:Chromatography was first invented by M. Tswett a botanist in 1906 in Warsaw, for the separation of coloured substances into individual components. Nowadays it is used to separate almost any given mixture, whether coloured or colourless, into its constituents. The more chromatography means colour writing. (Chroma = colour and Graphy = writing). This technique is based on the difference in the rate at which the components of a mixture move through a porous medium (stationary or fixed phase) under the influence of some solvent or gas (mobile phase). CLASSIFICATION:Based on the nature of the fixed or moving phase, chromatography is classified as (A) Adsorption Chromatography: It is based on the differences in the adsorption coefficient. In this, the fixed phase is a solid e.g. alumina, magnesium oxides, silica gel etc. The solutes are adsorbed in different parts of the adsorbent column. Adsorbed components are then eluted by passing suitable solvents through the column. (B) Partition Chromatography: The fixed phase may be a liquid strongly adsorbed on a solid. The solute gets distributed between the fixed liquid and the moving liquid. Paper chromatography is a special case of partition chromatography in which the adsorbent column is a paper. (C) Gas Chromatography: When moving the phase is a mixture of gases, it is called gas chromatography or vapour phase chromatography. (VPC.) PAPER CHROMATOGRAPHY PRINCIPLE: It is a part of chromatography. The substances are distributed between two liquids. Stationary liquid is held in the fibers of the paper and moving liquid is a solvent. The components of mixture migrate at different rates and appear as spots at different points on the paper. DEPARTMENT OF CHEMICAL SCIENCES NVPAS
2 Originally it was used to separate mixture of organic substances. But now it is used to separate cations and anions of inorganic substances as well. In this technique, a drop of test solution is applied as a small spot on the filter paper and the spot is dried. The paper is kept in a closed chamber and the edge of the filter paper is dipped into a developing solvent. When the liquid reaches the spot of the sample, the various substances are moved by the solvent at different speeds. When the components are moved to a suitable height, the paper is dried and the various spots are visualized by visualizing reagents. The movement of substances relative to the solvent is expressed in terms of migration parameters.
MIGRATION PARAMETERS: Migration parameters indicates the position of migrated spots on the chromatograms , RF, RX , RM and RC These parameters are also qualitative and quantitative. RF:- The R is related to the migration of the solute front relative to the solvent front. Distance traveled by solute from origin line RF = Distance traveled by solvent from origin line. RC is a function of partition coefficient. It is a constant for given substance. It may very with respect to temperature, type of paper, duration and direction of development, nature and the shape and the size of wick used, the amount of liquid in reservoir, humidity etc. The RF value depends upon the following factors: 1) The solvent employed 2) The medium used for separation (e.g. quality of paper) 3) The nature of the mixture 4) The temperature 5) The size of the vessel. Keeping all above factors constant, it is possible to compare the RF values of different substances. In some cases, the solvent front runs off the end of paper, in such case the movement of a substance is expressed as RX.
RX =
Distance traveled by substance from origin line. Distance traveled by standard substance from origin line.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
RM : According to Bate Smith and Westall, RM is defined as 1 RM = log 1 RF RM is additive and is compared of the partial RM values of individual functional groups and other groupings of atoms in the molecules. TYPES OF PAPER CHROMATOGRAPHY: YPES 1) Descending Chromatog Chromatography: When the development of the paper is done by allowing the solvent to travel down the paper, it is known as descending technique. The apparatus is a well sealed glass tank of suitable size and shape. It is provided with a trough for the mobile phase in the upper portion. The paper with the sample spotted is inserted in the trough containing the mobile phase. The advantage of descending technique is that the development can be c continued even though the solvent runs off the other end of the paper.
Fig. Descending Chromatography 2) Ascending Chromatography Chromatography: When the development of the paper is done by allowing the solvent to travel up the paper, it is known as ascending techniqu technique. Mobile phase is placed in a suitable container at the bottom of the chamber. DEPARTMENT OF CHEMICAL SCIENCES NVPAS
4 The samples are applied a few centimeters from the bottom edge of the paper suspended from a hook. (Fig a) (FigAlternatively, the paper may be rolled into a cylinder held together by staples, strings cylinder, r or plastic clips. Both, ascending and descending techniques are used for separation of organic and inorganic substances. The descending technique is preferred if the RF values of various constituents are almost same.
Fig. Ascending Chromatography
3) Ascending-Descending Chromatography: Descending It is the hybrid of the ascending chromatography and descending chromatography. chromatography In this technique the upper part can be folded over a glass rod allowing the ascending development to change o ding over into the descending after crossing the glass rod.
Fig. Ascending Descending Chromatography
4) Radial Chromatography hromatography: It is also known as circular chromatography. Radial development of solvent takes place. Circular filter paper is used. Materials to be analyzed are place at its centre. s After the drying the spots the paper is fixed horizontally on the Petri dish. Petri possess developing solvent. The tongue or wick of the paper is dipped in the solvent. Cover the paper by means of Petri Petri-dish cover. The solvent rises through the wick.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
5 The components get separated in the form of concentric circular zones.
Fig. Radial Paper Chromatography
5) Two Dimensional chromatography: wo A square or rectangular paper is used. Sample is applied to one of the corner. Solvent is developed. The second development is performed at the right angle of the direction of the first run. Identical solvent systems are used in both directions.
Fig. Two Dimensional Paper Chromatography EXPERIMENTAL DETAILS FOR QUALITATIVE ANALYSIS: QUALITATIVE 1) CHOICE OF TECHNIQUE: The choice of technique depends upon the nature of the substances to be separated. 2) CHOICE OF FILTER PAPER: The choice of filter paper depends on the type of problem under investigation. The following factors govern the choice. a) Whether the paper is being used for quantitative or qualitative analysis. b) Whether it is used for analytical or preparative chromatography. DEPARTMENT OF CHEMICAL SCIENCES NVPAS
6 c) Whether the substances used are hydrophilic or lipophilic, neutral or charged species. Various types of Whatmann chromatographic paper are available Generally coarser and faster papers are used when substances to be separated have wide-apart RF values. Slow papers are rarely used. Better resolution of substances with close RF values can be obtained. Heavy papers are used for preparative purposes. Whatmann filter papers have a content of 99% of - cellulose. The rest is mineral content. For the efficient separation of polar substances, the exchange capacity of the paper is increased by increasing the carboxyl content by partial oxidation. The capillarity of the paper can be increased by soaking it in 7% hydrochloric acid for 24 hours and washing successively with water and ethanol. It is possible to increase the rigidity of the paper by acetylation, esterfication and other chemical methods. 3) Proper Developing Solvent: The best possible developing solvent is generally selected. It depends on the fact that RF values should be different for different constituents present in a mixture. Generally a solvent which gives a RF of 0.2 0.8 for sample should be selected. If a pure solvent is not satisfactory, mixture of various with suitable polarity may be used. 4) Preparation of Samples: There is no specific method for preparation of samples. However, the sample volume of 10.2l having as many g of the substance is the ideal quantity to be spotted.
5) Spotting: A horizontal line (origin line) is drowning on the filter paper by a lead pencil. On the origin line, cross marks are made 2 cm away from each other. By the help of a graduated micro pipette the test solutions are applied on cross ( ) marks and the spots are dried by hot or cold air. In fig. A, B, C and D are four cross marks. On A, B and C solutions of pure substances are applied. On D, the mixture of a, B and C is applied.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
6) Drying the chromatograms: The Wet chromatograms after development are dried in special cabinets heated electrically with temperature control. 7) Visualization: There are two ways: a) Chemical Detection chromogenic (Visualizing) reagents are used to develop Detection: lizing) the colour of colourless spots. The regents are applied by pressure spraying or by dipping the chromatogram. Regents in organic solvents are more suitable. b) Physical Methods UV lamp fluoresces the colourless spots. If the substance Methods: f is coloured, the spots can be observed either by reflected or transmitted light. 8) Calculation of RF Values: The distance of chromatographic spot from its centre to origin line and the distance of solvent front from the origin the gives t RF values. the EXPERIMENTAL DETAILS FOR QUANTITATIVE ANALYSIS: The preliminary separation is same as qualitative analysis. Then, the assay can be performed either after extraction from the paper or in situ on the paper. 1) Estimation after extraction from the paper: I. Isolation of separated components from paper: The simplest procedure is to cut out the appropriate part of the filter paper having spot and to soak it in the minimum quantity of the solvent. Generally a semi micro extractor is being used. The separation of substance from the spot is separation known as elution. II. Determination: The microanalysis of the resultant eluate can be performed by Gravimetric estimation, UV Spectrophotometry, colorlmetry, Fluorimetry, Polarography, Coulometry, Radioactivity, Flame p photometry. The following information is required in the assessmenty of the merits of any procedure. a) The nature of the substance to be analyzed. b) The scientific equipment available and its sensitivity c) The time available, and ilable, d) The alternative metho available. methods DEPARTMENT OF CHEMICAL SCIENCES NVPAS
2) In situ methods: i. By visual assessment: To see the spot human e.g. it is not very quantitative. ii. By measurement of areas: The size of the spot may serve for determining the quantity of the substance. There is possibility of random errors due to variation of the spot shape. The volume of applied sample and the speed of application should be identical in all cases. iii. By densitometer: Intensity of the colour of a substance is measured directly on a chromatogram. iv. Potentiometry: Quadrant electrometer or electronic voltmeters are used to measure changes in potential of a metal electrode in contact with the filter paper. Other suitable methods are conductivity, absorptiometry, polarography, etc. APPLICATIONS: 1. Paper chromatography has widely been used for quantitative analysis of inorganic, organic and biochemical interest. 2. As a check on process: It has also been used for checking of the other separation methods and purification processes. 3. Paper chromatography is ideally suited for rapid analysis of reaction mixture and so it is versatile tool in the hand of organic chemists. 4. Paper chromatography has been successfully used for characterizing and isolating the following organic compounds. Acids, Alcohols, Glycols, Alkaloids, Amines, Amino acids, proteins and peptides, Antibiotics etc. 5. Paper chromatography has also been used in the analysis of mixture of sugars.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
9 THIN-LAYER CHROMATOGRAPHY INTRODUCTION: This technique was first introduced in 1938 by Izmailov and Shraiber. It was used to separate plant extracts on 2 mm thick and firm adhesive layer of alumina set on glass plates. Consden, Gorden and Martin (1944) started using filter papers. They worked in the field of amino acid analysis. Williams carried out chromatography on adsorbent layers. Sandwitched between two glass plates. One of the glass plates has a small hole through which solutions and developing solvents were applied. Kirchner developed adsorption chromatography on impregnated filter paper and glass fiber paper coated with silicic acid or alumina. TLC as an analytical procedure was introduced by Stahl in 1958. He was mainly responsible for bringing out standard equipment for preparing thin layers. TLC is often named as drop, strip, spread layer surface chromatography and open column chromatography. Superiority of TLC over other chromatographic Techniques:The main advantages of TLC are listed below: i. Simple equipment. ii. Short development time of about 1 hour. iii. Wide choice of stationary phases and hence it may be employed for adsorption, partition or ion-exchange chromatography. iv. Early recovery of separated components. v. Separation effects are usually superior to those obtained by paper chromatography. vi. Detection of fluorescence compounds under UV lights is easier than on paper. vii. Extremely sharp delineated spots are obtained in TLC. viii. Variable thickness of thin layers: The methods may also be applied for the preparative separation with the aid of thicker layers. ix. The greatest merit of TLC is the use of chemically inerl stationary phase to afford vigorous means of detection including the application of strong heat and corrosive reagent such as conc.H2SO4. Experimental Technique:1) Coating Materials: A large no. of coating materials is known. E.g. Silica gel, alumina, cellulose powder etc. Other materials are calcium phosphate, magnesium trisilicate, polyamide, Silica gel-alumina (1:1), acetylated cellulose, ferric oxide hydrate etc. Adsorbents do not adhere very satisfactory to glass plates. To overcome this problem, some binders like gypsum, starch etc. are added to adsorbents. DEPARTMENT OF CHEMICAL SCIENCES NVPAS
10 Adsorbents are called by names such as silica gel-G, Alumina-G etc. The G letter G indicates the presence of the binder gypsum. An inert fluorescent indicator is added to coating materials. The thin layer e thus produced fluoresces on exposure to UV light. Thus the non fluoresces but light. UV adsorbing substances can be detected. These substances show up as dark spots. While choosing adsorbents: The characteristic if the compound should be known. The solubility and its basic, acidic or amphoteric nature must be checked. The chemical reactivity of compound with adsorbent should be known. 2) Preparation of thin layers on plates: The thin layer should be consistent throughout. The coating material is used in the form of a suspension or slurry in some used liquid in most of the methods. The various methods are as follows: i. Pouring: A measured amount of the slurry is put on a given sized plate and the plate is then tipped back and forth to spread the slurry unifo uniformly over the surface surface. ii. Dipping: Plates are prepared by dipping them at a time in chloroform or chloroform- methanol slurries. iii. Spraying: A small point sprayer was used. This technique is not used now a day because it is difficult to obtain uniform layers, on a single plate and on also there may be variation from plate to plate plate. iv. Spreading: The slurry is placed in an applicator. This is either moved over the stationary phase or it is held stationary and the plate is pushed or pulled through.
v.
Fig. Spreading The apparatus consists of two parts: the aligning tray in which the plates are set in a line, and spreader. An improved TLC spreader, the layer thickness can be adjusted 0.0 to 2.0 mm.The spreading method is the most important now a days. Precoated plat ecoated plates: Precoated plastic or glass sheets are available. These plates are quite expensive. But there is no need to purchase expensive coating equipments. ing
3) Activation of adsorbent: After coating the liquid associated with thin layer is removed by drying the thin layer plate for 30 minutes in air and then in the oven at 110oc for another 30 minutes. This makes the adsorbent layer active. DEPARTMENT OF CHEMICAL SCIENCES NVPAS
11
In order to obtain very active layers, Silica gel and alumina plates can be heated Silica-gel o to 150 c for about 4 hours. 4) Purification of Silica-gel G layers: gel layers:Iron impurities present in silica gel `G` is removed by development with methanol conc. HCl (9:1 v/v). The iron gets migrated with the solvent front to the upper edge of the plate. The plates are again dried and activated at 1 oc. 110
5) Sample Application: Agla micro syringe is generally used for quantitative work. Capillary tubes may be used for the same. Solvents used for sample solution should be volatile and as non polar as possible. non-polar possible 6) Development Tank: The development tank is used in paper chromatography is also used in TLC. tank Ascending chromatography is the most common in TLC. In TLC, the plate is placed in a chamber at 45o angle. The bottom of the . chamber is covered up to 1 mm by the solvent. Three sides of the tank are lined with solvent impregnated paper. While top is covered tightly with the ined lid. The development chamber should be perfectly saturated with solvent vapour to avoid unequal solvent evaporation which can lead lack of reproducibility in RF values. Descending TLC is also possible. It has no advantage in terms of efficiency. LC Horizontal TLC is also possible. However, it finds little use.
Fig. Development of tank
7) Solvent system: If one does not know about the nature of the components of the mixture, the best eluent is found by trial and error using small, very rapid running TLC t small, plates. If one knows the chemical nature of the solutes to be separated, it is possible to select a suitable solvent by using Stahls triangle.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
12 Mixtures of two or more solvents of different polarity often give better separately. The solvents used in chromatography are as pure as possible. 8) Development Methods: Generally ascending technique is used. This is a quite quick process which requires 20-40 minutes. 9) Detection of Components: One can see coloured substances usually. Colourless components can be detected under UV light or by treating with a visualizing reagent. The corrosive visualizing reagents can be used. 10) Evaluation of chromatogram: (A) Qualitative: This is same as with paper chromatograms. (B) Quantitative: The quantitative evaluation can be carried out in two ways: I. Direct Methods: 1) Visual assessment of chromatograms: The only detector is the human eye. 2) Determination by measurement of spot area: The method is based on a mathematical relationship between the spot area and the amount of substance. 3) Densitometry: The intensity of the colour of a substance is measured directly on chromatogram. 4) Direct spectrophotometry on chromatogram: Wavelength of maximum absorption is measured. IR spectroscopy, reflectance spectroscopy, spark chamber method etc. are additional methods. II. Indirect Methods: This involves recovery of the chemical constituents by elution and subsequent determination with a suitable instrument. These methods are known as elution techniques. In this method elution of the solute from the adsorbent is done by simple agitation with a solvent followed by the removal of adsorbent by centrifugation. The micro analysis of the resultant eluate can be done by one or more of the following techniques: (a) Gravimetric, (b) UV spectrophotometry, (c) Colorimetry, (d) Fluorimetry (e) Polarography, (f) Coulometry, (g) Radiometry, (h) Flame photometry (i) Vapour phase chromatography etc.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
13 In the analysis, the following information is required i. The nature of the substance ii. The equipment and its sensitivity iii. The time available and iv. The alternative methods available and their relative accuracy. As compared with direct methods, the elution technique requires more time and certain amount of substance remains unrecoverable. The greatest advantage of the elution method has a very low value of random errors as compared with direct method. Application of TLC:1) As a check on process: It has been used for checking of the other separation procedures and purification processes. 2) In Organic Chemistry: The main use of TLC is isolation and separation of individual components of a mixture. The main reasons for popularity of TLC as an analytical and preparation methods are: i. It can be used for most of chemical compounds. ii. It has high speed of separation. iii. It `s selectivity is high. The following are the various applications of TLC in organic chemistry: (a) For checking the purity samples. (b) As a purification process. (c) Examination of reactions. (d) For identifying organic compounds. TLC has been successfully used for characterizing and isolating the following organic compounds. i. Acids ii. Alcohols iii. Glycols iv. Alkaloids v. Amines vi. Amino acids, proteins and peptides vii. Antibiotics Besides these, there are compounds like carbohydrates, carbonyl compounds, Dyes, Hydrocarbons, lipids, nucleic acids, pesticides, natural pigments, pharmaceutical products, phenols, steroids, terpenes, essential oils, vitamins, adhesives, explosives plasticizers etc. which have been separated and characterized by TLC. 3) For separation of Inorganic Ions: Recently TLC has been used for separating cationic, anionic, purely covalent species and also some organic derivatives of the metals.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
14 In order to carry out TLC of groups of cations, silica gel is first washed with acid and coater to remove impurities of sodium, magnesium, calcium and iron. But this treatment removes the calcium sulphate binder. Therefore, calcium sulphate must be replaced by starch and other suitable binder. 4) Applications of TLC in quantitative analysis: i. ii. iii. iv. v. vi. vii. viii. ix. Spectrophotometric Measurement Fluorimetric Method Spectral reflectance Visual comparison of spots Spot area and weight relationship Spot densitometer Vapour phase chromatography Radioactive methods Volumetric analysis Limitation:o The main limitation of TLC is that it is used only for small scale preparative work. o High resolution can be obtained by using column chromatography or film technique. Limited successes have been achieved in both the cases. Scope:o Due to its inconvenience, simplicity, sharpness of separations, high sensitivity, speed of separation and case of recovery of separated compounds, TLC is very useful in all branches of chemistry. o It is a versatile process in analytical investigations of the substances which are available in small quantities. o It will be greater utility in biological and chemical investigations.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
15
COLUMN CHROMATOGRAPHY PRINCIPLE:The principle of selective adsorption (i.e the rate of adsorption (i.e. varies with a giv adsorption varies with a given adsorbent for given ption different material) is used in column chromatography. In this method, the mixtures to be separated are dissolved in a suitable solvent and allow to pass through a tube containing the adsorbent. The component having greater adsorbing power is adsorbed in the upper part of the column. The next component is adsorbed in the lower part of column. As a result the material are partially separated. materials The initial separation can be improved by passing suitable solvent slowly through the column. The various bands presents in the column become more defined defined. The banded column of adsorbent is termed as a chromatogram. The portion of a column which is occupied by a particular substance is called its zone. In order to separate or to estimate the various constituents, two procedures may be adopted: i. After development, the column of adsorbent may be pushed out of the tube, the various zones are cut with a knife at boundaries and the substance present in zones extracted with suitable solvent. This process is known as elution. suitable ii. After development, the column may be washed with more solvent and each component is collected separately at the end of the column.
Fig. Elution development of two solutes A and B. E is eluent. Her A is more strongly adsorbed than B
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
16
EXPERIMENTAL DETAILS: DETAILS:1) Apparatus:a simple straight glass tube tapered at the bottom and often fitted with a top is commonly used. The ratio of length to diameter should be greater than 20:1; a ratio 40:1 can be taken as standard. This tube is about 20- cm long and 2-3 cm in diameter. -30 This may hold 50-100 gm of adsorbent and may retain several grams of adsorbate. 100 The adsorbent is supported on a plug of cotton or glass wool, for wider tubes a perforated disc, covered by a pad of cotton or glass wool may be employed. Long and narrow tubes are used for difficult separation. Columns fitted with cooling jackets are used when constant temperature conditions are required.
2) Adsorbents:The adsorbents should have the followi characteristics: following a. Their particles should be spherical in shape and uniform in size. b. Their mechanical stability must be great enough to prevent the deposition of fine dust in the channels of the packing. c. They should not react chemically d. They should contain as small amount of soluble components as possible. in e. They should be catalytically inactive. e.g. Silica-gel, activated charcoal, magnesium oxide, magnesium carbonate, gel, calcium carbonate, barium carbonate, etc. 3) Preparation of Adsorption Columns: Columns:The glass wool or cotton plug is used as a support for the column. It is first kept in ss position in the tube. The tube is then clamped vertically. Now the adsorbent is ads added in portion so that the tube is packed uniformly with the adsorbent. Whenever any portion i added, it is pressed from above with a flattened glass rod is before the next portion is added. This is continued till nearly two thirds of it is filled. An alternative procedure involves the preparation of slurry of the adsorbent in a suitable liquid medium. Liquid may be petroleum either or sometimes the same medium. solvent which is being used in the chromatographic process. Now the slurry is
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
17 added to the tube gradually. The best method is to connect the tube to a suction pump through the stopcock. Suction is applied after each addition. This process is continued till a column of desired height is obtained. It is important to remember that the surface of the column should remain covered with petroleum either all along. 4) Solvents used:The choice of solvent depends upon the solubility relations of substances. Low boiling point (40o-80oC) solvents are generally used. E.g. light petroleum, cyclohexane, Carbon disulphide, benzene, chloroform, CCl4, ethyl acetate, methyl chloride, ethanol, acetone, ether and acetic acid. The solvents used in chromatography have three functions to perform: i. They serve to introduce the mixture to the column. ii. They are used to develop the chromatogram to their fullest extent. iii. They are used as eluents. A good eluent must be a liquid which can dissolve readily the various components from the column. 5) Detectors:There are used to monitor to determine quantitatively the dissolved substances emerging from the column. The various detectors are as follows:I. Optical Detectors: - Old type of flow analyzers. - Small cells made from glass and quartz. - Used for continuous photometric analysis with visible or UV light. II. Differential Refractometers: - based on refraction of the emerging power - Limit of detection 10-8 g. III. Detectors based on heat of adsorption: - Known as micro adsorption detectors. - There are two cells, one located on top of the other. The lower cell is filled with an adsorbent - The liquid emerging from the column is passed through two cells. IV. Flame Ionization Detectors V. Conductivity Detectors:- The effluent is passed through the measuring cell. - Cell contains two or three platinum electrodes with in a Wheatstone bridge circuit. 6) Methods of introducing the solution:The top of the column is covered by a plug of cotton wool. Some solvent is put over it through a funnel. The rate of the flow of solvent through the column is controlled by suction pump. Now the solution is added through a funnel. The top of the columns must remain covered with liquid from the introduction of the first liquid to the end of development. If this precaution is not taken into account, the column may dry and shrink. Too much suction should be avoided to prevent evaporation.
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
18 After the entire portion if the solution has passed through the developing liquid is led into column slowly. After all the liquid has passed through and various zones become well defined, the system is allowed to dry in air or oxygen. 7) Analysis:The adsorbent may be pushed out completely with the wooden pestle or plunger and the zones may be separated as they leave the tube. Each zone is then dropped immediately into the eluent and the suspension is filtered. In same cases, the column of adsorbent is not removed from the glass tube. The developed chromatogram is treated either with a single eluent or with a succession of solvents having increasingly powerful eluents action. The various portions of the column are then washed out one by one and collected in different receivers. If the separation is not complete, the extracts are allowed to pass through the adsorbent column. The series of fractions collected in this method constitutes the liquid chromatogram. Factors Affecting Column Efficiency:I. Nature of solvents: Solvents of low viscosities are generally used. Lower the viscosity higher the rate of flow. II. Dimension of column: It is possible to imp rove the column efficiency by increasing the length/width ratio of the column. III. Particle size column packing: It is possible to increase the column efficiency by decreasing the particle size of the adsorbent. The usual particle sizes range from 100 to 200 meshes. IV. Pore diameter of column packing: Generally, polar adsorbents posses a pore diameter of 20Ao. V. Temperature of the column: Difficult soluble samples are generally separated at higher temperatures while other samples are separated at room temperature. Application of Column Chromatography:1) Analytical uses: For analytical purposes, column chromatography finds limited applications. Vestergaard and Sayegh could separate seven urinary steroids within 5 hours which requires 36 hours on a normal column. They have used narrow Teflon tubing packed with aluminum oxide or silica gel. 2) Separation of geometrical isomers: The separation of cis/trans isomer is based on the steric factors. Isomers whose functional groups can approach the surface of the adsorbent more easily are more strongly adsorbed. 3) Separation of Diastereomers: 4) Separation of tautomeric mixtures: 5) Separation of racemates:
DEPARTMENT OF CHEMICAL SCIENCES
NVPAS
You might also like
- Paper ChromatographyDocument34 pagesPaper ChromatographyShaise Jacob100% (6)
- Chemistry ProjectDocument28 pagesChemistry ProjectVikramjeet Singh83% (12)
- Instrumental Methods of Drug AnalysisFrom EverandInstrumental Methods of Drug AnalysisRating: 3 out of 5 stars3/5 (3)
- NUS ME4245 Part 2 Quiz Tutorial SolutionsDocument20 pagesNUS ME4245 Part 2 Quiz Tutorial SolutionsYogesh ParthasarathyNo ratings yet
- Tutorial#2Document10 pagesTutorial#2ahmad karisNo ratings yet
- Paper Chromatography 2023 - 230901 - 173633Document35 pagesPaper Chromatography 2023 - 230901 - 173633p.ishaanpawarNo ratings yet
- Chromatographic Techniques 1200328060603035050Document12 pagesChromatographic Techniques 1200328060603035050KIRAN ALLUNo ratings yet
- Analytical Techniques Final Note NADocument121 pagesAnalytical Techniques Final Note NAyuvi78312No ratings yet
- Paper & Gas ChromatographyDocument21 pagesPaper & Gas ChromatographyusamaNo ratings yet
- Instumental Methods of AnalysisDocument10 pagesInstumental Methods of AnalysissanjhinehrotraNo ratings yet
- Experiment 2Document3 pagesExperiment 2Ushna Asif BSCHE-ENo ratings yet
- SBCC 1106-2Document17 pagesSBCC 1106-2opolla nianorNo ratings yet
- Lecture 04 05Document38 pagesLecture 04 05Munna IslamNo ratings yet
- HrmtograhiDocument8 pagesHrmtograhiALJOREY LAZARITONo ratings yet
- Chapter 3.4 Paper Chromatography FinalDocument31 pagesChapter 3.4 Paper Chromatography FinalShalik RazaNo ratings yet
- CHEMDocument13 pagesCHEMMohamed MusthapaNo ratings yet
- Principle of ChromatographyDocument8 pagesPrinciple of ChromatographyMuhammad ZeeshanNo ratings yet
- ChromatographyDocument11 pagesChromatographyAmrit KoiralaNo ratings yet
- Principle of Paper Chromatography: The Separation in Paper Chromatography Is AchievedDocument12 pagesPrinciple of Paper Chromatography: The Separation in Paper Chromatography Is AchievedSoumik MahapatroNo ratings yet
- IMA Lecture 13Document3 pagesIMA Lecture 13Shahrukh SindhiNo ratings yet
- Chromatography and Paper ChromatographyDocument29 pagesChromatography and Paper Chromatographymostafa33puvmNo ratings yet
- Air Force School Ambala CanttDocument16 pagesAir Force School Ambala Canttsimran ranaNo ratings yet
- Chromatography 1Document81 pagesChromatography 1adarshthakur41973No ratings yet
- Paper ChromatographyDocument5 pagesPaper ChromatographyKarishmaNo ratings yet
- Instrumental Analysis IDocument151 pagesInstrumental Analysis IYona KelbessaaNo ratings yet
- Chromatography P1eeaoqbpea91bc5e2b1cc84Document91 pagesChromatography P1eeaoqbpea91bc5e2b1cc84Asif AliNo ratings yet
- ChromatographyDocument88 pagesChromatographyMohammad Sabir HussainNo ratings yet
- Paper Chromatography-1Document29 pagesPaper Chromatography-1gayatri maldhureNo ratings yet
- Chapter 18 - ChromatographyDocument16 pagesChapter 18 - ChromatographyJames Miller100% (1)
- Chromatograph Y: Presenting To: Nishat Rayhana Lecturer Univrsity of Development AlternativeDocument41 pagesChromatograph Y: Presenting To: Nishat Rayhana Lecturer Univrsity of Development AlternativeamolNo ratings yet
- Paper Chromatography Notes FinalDocument8 pagesPaper Chromatography Notes FinalBijayaKumarUpretyNo ratings yet
- Chemistry Project Paper ChromatographyDocument20 pagesChemistry Project Paper ChromatographyAmrita S100% (1)
- Exercise 4 (Chromatography)Document6 pagesExercise 4 (Chromatography)fangirlton0% (1)
- Planner Chromatography by Miss IshratDocument13 pagesPlanner Chromatography by Miss IshratTuba AhmedNo ratings yet
- Cromatografia - Cap 621 UspDocument34 pagesCromatografia - Cap 621 UspMatias100% (1)
- Analytical Chemistery: Muhammad Azhar 1056 Bs Chemistry 4 (M)Document5 pagesAnalytical Chemistery: Muhammad Azhar 1056 Bs Chemistry 4 (M)Open UserNo ratings yet
- What Is ChromatographyDocument41 pagesWhat Is ChromatographyLaura EdwardsNo ratings yet
- Che ChromatographyDocument15 pagesChe ChromatographyArpit MauryaNo ratings yet
- Physical Tests / Á621ñ Chromatography 1Document10 pagesPhysical Tests / Á621ñ Chromatography 1Villalpando D. PabloNo ratings yet
- 37 Analytical Techniques PDFDocument225 pages37 Analytical Techniques PDFKomal EhsanNo ratings yet
- Experiment 3Document10 pagesExperiment 3bharathshemfordvnb18No ratings yet
- ChromatographyDocument10 pagesChromatographyBioclass BitesNo ratings yet
- DocumentDocument21 pagesDocumentcᴘcтԍᴀмιɴԍ YTNo ratings yet
- Unit 5 ChromatographyDocument86 pagesUnit 5 ChromatographyRujal KundhareNo ratings yet
- ChromatographyDocument6 pagesChromatographyAyush K. SharmaNo ratings yet
- Unit-4 Chromatography PDFDocument9 pagesUnit-4 Chromatography PDFhacker7899No ratings yet
- Chromatography SLT SpyDocument6 pagesChromatography SLT SpyAbdulnafiu AhmadNo ratings yet
- Cromatografia - Cap 621 Usp PDFDocument34 pagesCromatografia - Cap 621 Usp PDFMatias OliveraNo ratings yet
- SBCC 1106-1Document30 pagesSBCC 1106-1opolla nianorNo ratings yet
- Biochem Lab Report 1Document7 pagesBiochem Lab Report 1AeeshaNo ratings yet
- Principles of PC, TLC and HPLC andDocument5 pagesPrinciples of PC, TLC and HPLC andjust-maybe202No ratings yet
- Paper ChromatographyDocument6 pagesPaper ChromatographysamNo ratings yet
- Exercise 4 (Chromatography)Document6 pagesExercise 4 (Chromatography)Wendell Kim LlanetaNo ratings yet
- Biochem LabDocument6 pagesBiochem LabSyed Sharique AkhtarNo ratings yet
- SEMESTER 3 Practical Science 2 Experiment 8 TopicDocument6 pagesSEMESTER 3 Practical Science 2 Experiment 8 Topickat tunNo ratings yet
- Chromatography: Presenting To: Nishat Rayhana Lecturer Univrsity of Development AlternativeDocument41 pagesChromatography: Presenting To: Nishat Rayhana Lecturer Univrsity of Development AlternativeamolNo ratings yet
- Usp 621 PDFDocument7 pagesUsp 621 PDFnur_yusri_1No ratings yet
- Paper ChromatographyDocument4 pagesPaper ChromatographySai SridharNo ratings yet
- Systematic Methods of Water Quality Parameters Analysis: Analytical MethodsFrom EverandSystematic Methods of Water Quality Parameters Analysis: Analytical MethodsNo ratings yet
- Faria Et Al-1998-Journal of Geophysical Research - Oceans (1978-2012) PDFDocument16 pagesFaria Et Al-1998-Journal of Geophysical Research - Oceans (1978-2012) PDFAnonymous 7oIDoGjNo ratings yet
- Impurity of Mg2Si - AlDocument7 pagesImpurity of Mg2Si - AlJasonLopezNo ratings yet
- Aerodynamic Modeling & Simulation of HGVDocument26 pagesAerodynamic Modeling & Simulation of HGVManjunath PattarNo ratings yet
- Tutorial For Random Variables and Stochastic Processes Eem2046 Engineering Mathematics Iv Multimedia UniversityDocument9 pagesTutorial For Random Variables and Stochastic Processes Eem2046 Engineering Mathematics Iv Multimedia UniversityNazmi AzizNo ratings yet
- Dispersión de Contaminantes (NO) : Cabrera Araujo Liliana AndreinaDocument10 pagesDispersión de Contaminantes (NO) : Cabrera Araujo Liliana AndreinaLiliana AndreinaNo ratings yet
- Neet Test Series 2022 Test Code: NT - 12: Biology Physics ChemistryDocument23 pagesNeet Test Series 2022 Test Code: NT - 12: Biology Physics Chemistry꧁༒ŞɨĐĐHÄRŦ卄༒꧂ ᴊʜᴀ࿐No ratings yet
- Paper 11 Sem-5 Vbu & Bbmku Guess PaperDocument37 pagesPaper 11 Sem-5 Vbu & Bbmku Guess Papernnbhbbb83No ratings yet
- Common Modes of Dynamic BehaviorDocument15 pagesCommon Modes of Dynamic BehaviorDennis T. Beng HuiNo ratings yet
- Periodic TableDocument6 pagesPeriodic TableSamantha AceroNo ratings yet
- Asked By: AshleyDocument2 pagesAsked By: Ashleyir123No ratings yet
- Determining The Izod Pendulum Impact Resistance of Plastics: Standard Test Methods ForDocument20 pagesDetermining The Izod Pendulum Impact Resistance of Plastics: Standard Test Methods ForMeethaq AbedNo ratings yet
- Me 2204 - Fluid Mechanics and MachineryDocument3 pagesMe 2204 - Fluid Mechanics and MachineryKarthik SubramaniNo ratings yet
- Ial Maths Pure 4 CR5Document10 pagesIal Maths Pure 4 CR5nasehaNo ratings yet
- Section 4 Non-Rigid Transformations, Congruence, and Similarity (Workbook)Document20 pagesSection 4 Non-Rigid Transformations, Congruence, and Similarity (Workbook)jazzyNo ratings yet
- Diloxanide Furoate Profile PDFDocument37 pagesDiloxanide Furoate Profile PDFawdawdawd6004No ratings yet
- CylcotronDocument14 pagesCylcotronhey707070100% (2)
- Bobrick 2021 Class. Quantum Grav. 38 105009Document23 pagesBobrick 2021 Class. Quantum Grav. 38 105009MaxNo ratings yet
- Professor Jean-Louis Basdevant Auth. Variational Principles in PhysicsDocument190 pagesProfessor Jean-Louis Basdevant Auth. Variational Principles in PhysicsAbdellah AzzouzNo ratings yet
- Class 10000 SD and HD Owners Manual - TMC - 704PDocument16 pagesClass 10000 SD and HD Owners Manual - TMC - 704Poserravalle70No ratings yet
- GR InsDocument4 pagesGR InsMohan Raj JercyNo ratings yet
- Ordering Information Page 1: Ordering Information / Specification Sheet For Circular Bellows Expansion JointDocument6 pagesOrdering Information Page 1: Ordering Information / Specification Sheet For Circular Bellows Expansion JointmishtinilNo ratings yet
- Intended ActivityDocument2 pagesIntended Activitylily flowerNo ratings yet
- Magnetism and Matter - 231006 - 224401Document13 pagesMagnetism and Matter - 231006 - 224401patelsur2No ratings yet
- Pp04 - Asep - NSCP 2015 Update On Ch2 Section 207 Wiind LoadsDocument169 pagesPp04 - Asep - NSCP 2015 Update On Ch2 Section 207 Wiind LoadsCarlo Joseph88% (8)
- Serway 6 e Problems 32Document14 pagesSerway 6 e Problems 32Antoni HCNo ratings yet
- Using A Matlab Implemented Algorithm For Uv-Vis SpectralDocument7 pagesUsing A Matlab Implemented Algorithm For Uv-Vis SpectralCesar FrancoNo ratings yet