Professional Documents
Culture Documents
HALOAKENES
HALOAKENES
Uploaded by
sivaranjini S.VCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HALOAKENES
HALOAKENES
Uploaded by
sivaranjini S.VCopyright:
Available Formats
Assignment
Chapter 10: Haloalkanes and Haloarenes
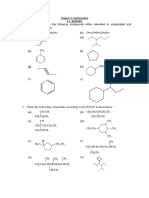
1. Name the following halides according to IUPAC system:
(a) CH3CH(Br)CH=C(CH3)CH2Cl (b) C(CH3)2Br
(c) CH3CH(CH3)CH(Br)CH3 (d) ClCH2C=CCH2Br
(e) Cl----- (f) CH3CH ≡ CI
2. What happens when bromine attacks CH2=CH-CH2-C ≡ CH ?
3. Write the structures of the following organic compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 2-(2-Chlorophenyl)-1-iodo octane
(iv) 4-tert-Butyl-3-iodoheptane
4. Answer the following questions:
(i) What is meant by chirality of the compound? Give an example.
(ii) Which of the following compounds is more easily hydrolysed by KOH and why?
CH3CH(Cl)CH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes SN2 faster and why?
I Cl
Or
5. Which one of the following reacts faster in an SN1 reaction and why?
Cl
Cl or
6. State one use of DDT and iodoform. Why chloroform is kept in dark coloured bottles
completely filled?
7. What are ambident nucleophiles? Explain with the help of an example.
8. Write short notes on:
(a) Fittig reaction (b) Swartz reaction
9. Account for the following:
i) tert-Butyl chloride reacts with aqueous NaOH by SN1 mechanism while n-butyl chloride
reacts by SN2 mechanism.
ii) Among HI, HBr and HCl, HI is most reactive.
iii) Alkyl halides though polar, are immisible with water.
iv) Chlorobenzene is extremely less reactive towards nucleophillic substitution reaction.
10 Carry out the following conversions:
i) 1-Chlorobutane to n-octane
ii) Toluene to benzyl alcohol
iii) Benzyl chloride to benzyl alcohol
11. What will be the mechanism for the substitution of -Br by –OH in (CH3)2C(Br)CH2CH3?
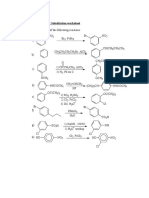
12. Identify the following compounds from A to T:
acetone
(a) CH3CH2CH2Cl + NaI A A
heat
(b) (CH3)3CBr + KOH Ethanol, heat B
( c) CH3CH(Br)CH2CH3 + NaOH (aq) C
ethanol
(d) CH3CH2Br + KCN D
(e) (CH3)3CBr + H2O heat E
C2H5ONa, heat
(f) (CH3)2CHCH(Br)CH2CH3 F
(g) CH3CH2Cl + SbF3 heat G
(h) CH2=CHCH2Br + H2O H
(i) C6H4CH2Cl + C2H5ONa I
(j) CH3CH2CH2OH + SOCl2 J
peroxide
(k) CH3CH2CH=CH2 + HBr K
(l) CH3CH=C(CH3)2 + HBr L
(m) Br
+ CH3NH2 M
CH2Br
Cl2, FeCl3
(n) OH N
(o) CH2CH2OH
+ HBr O
(p) CH(CH3)2 + Br2 heat P
You might also like
- A Level Chemistry Paper 1 Set 29 Marking GuideDocument7 pagesA Level Chemistry Paper 1 Set 29 Marking GuideSsenono AndrewNo ratings yet
- Anc Ment 638213274708770420Document16 pagesAnc Ment 638213274708770420shettisanjitNo ratings yet
- CH 44 Organic Reactions - Supp Ex 1 (Updated)Document4 pagesCH 44 Organic Reactions - Supp Ex 1 (Updated)伊貝P-No ratings yet
- Halo DerivativesDocument5 pagesHalo DerivativesBHASWATI MANDALNo ratings yet
- Hydrocarbons (Vijay B. Sir)Document69 pagesHydrocarbons (Vijay B. Sir)pritamkar721636No ratings yet
- ALCOHOL SHORT TEST-FINALDocument2 pagesALCOHOL SHORT TEST-FINALSparsh SinghNo ratings yet
- Section-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XDocument14 pagesSection-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XPriyansh YadavNo ratings yet
- CBSE Class 12 Chemistry Halo Alkanes and HaloareneDocument12 pagesCBSE Class 12 Chemistry Halo Alkanes and Haloarenedeepak vaishnavNo ratings yet
- Cls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Document34 pagesCls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Jotiraj Parihar50% (2)
- Level-1 Main (Encrypted)Document3 pagesLevel-1 Main (Encrypted)Adrian AlvaNo ratings yet
- PDF Alkanepdf DLDocument8 pagesPDF Alkanepdf DLGeraldineNo ratings yet
- CB and APEDocument4 pagesCB and APEAnubrata SarkarNo ratings yet
- ch8 1Document8 pagesch8 1yonggye100% (1)
- Aliphatic HydrocarbonsDocument11 pagesAliphatic HydrocarbonsRishabhNo ratings yet
- Haloalkanes and HaloarenesDocument18 pagesHaloalkanes and HaloarenesBhavesh KNo ratings yet
- Chapter 5.1Document3 pagesChapter 5.1Aliff AmirudinNo ratings yet
- Jee-Main ChemistryDocument5 pagesJee-Main ChemistryAbhishek SaravananNo ratings yet
- Chapter 10 Haloalkanes and HaloarenesDocument3 pagesChapter 10 Haloalkanes and HaloarenesAaryaNo ratings yet
- C - 8. (MAIN) (ALKYL & ARYL HALLIDE, ALCOHOL ETHERS & PHENOLS)Document10 pagesC - 8. (MAIN) (ALKYL & ARYL HALLIDE, ALCOHOL ETHERS & PHENOLS)revohe2640No ratings yet
- Chemistry - Sample Question Paper - 9Document6 pagesChemistry - Sample Question Paper - 9Mohd AdilNo ratings yet
- Safari - 24 Apr 2020 at 1:57 AMDocument1 pageSafari - 24 Apr 2020 at 1:57 AMAgatha chilesheNo ratings yet
- Electrophilic Aromatic Substitution Worksheet: CHEM 202Document2 pagesElectrophilic Aromatic Substitution Worksheet: CHEM 202SunnyNo ratings yet
- Orgsynth KeysDocument27 pagesOrgsynth KeysDebalina DassNo ratings yet
- Chemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Document7 pagesChemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Surya Charan Reddy100% (1)
- Hydrocarbons A 1Document4 pagesHydrocarbons A 1REJA MUKIB KHANNo ratings yet
- Sample Questions For Chemistry 2Bh/2Dh Multiple Choice Test THERMODYNAMICS Q1.Document4 pagesSample Questions For Chemistry 2Bh/2Dh Multiple Choice Test THERMODYNAMICS Q1.nihararmyNo ratings yet
- Organic Chemistry Test-1 On Total Syllabus: Single CorrectDocument5 pagesOrganic Chemistry Test-1 On Total Syllabus: Single CorrectVanshaj GuptaNo ratings yet
- ChemDocument6 pagesChembighneshrath1No ratings yet
- Xii Chem Q and A CH 10Document18 pagesXii Chem Q and A CH 10SÁMÃÑ KANNANo ratings yet
- Sample Paper: Time: 90 Minutes Max. Marks: 35Document9 pagesSample Paper: Time: 90 Minutes Max. Marks: 35Harsh PatelNo ratings yet
- Answer, Pre-Exam Practice Chem Sem 3 EssayDocument29 pagesAnswer, Pre-Exam Practice Chem Sem 3 EssayTing TCNo ratings yet
- 10 Hydrocarbons: AssignmentDocument6 pages10 Hydrocarbons: AssignmentalarmbarbarNo ratings yet
- 2024 Carbonyl Cpds Suggested SolutionDocument5 pages2024 Carbonyl Cpds Suggested SolutionMN4012022 CHIA CHANG YI, AARONNo ratings yet
- Mega Test (06-05-2024) - 240606 - 153839Document23 pagesMega Test (06-05-2024) - 240606 - 153839Regidrago 2No ratings yet
- Alkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHDocument17 pagesAlkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHEllaŠtrbacNo ratings yet
- Hydrocarbon Level:I KVPYDocument2 pagesHydrocarbon Level:I KVPYRAGHUL MNo ratings yet
- 10 Haloalkanes and Haloarenes: SolutionsDocument40 pages10 Haloalkanes and Haloarenes: SolutionsArpana100% (1)
- HaloalkeneDocument20 pagesHaloalkeneRashmi GuptaNo ratings yet
- Chemistry Class 12th CBSE Sample PaperDocument9 pagesChemistry Class 12th CBSE Sample PaperSiddhi GoplanNo ratings yet
- Alkyl Halides and Aryl HalidesDocument16 pagesAlkyl Halides and Aryl Halidesvardesh100% (1)
- Dated: 02-01-2019 TIME: 3 Hours M.M.: 360 (Full Test - 8) (Jee-Main)Document10 pagesDated: 02-01-2019 TIME: 3 Hours M.M.: 360 (Full Test - 8) (Jee-Main)Dikshit AroraNo ratings yet
- BB YASMt DVEvy ZZi PR NYRDocument8 pagesBB YASMt DVEvy ZZi PR NYRarindamNo ratings yet
- Alcohols, Phenols & Ethers (PDFDrive)Document144 pagesAlcohols, Phenols & Ethers (PDFDrive)vidya.bishtNo ratings yet
- OC04 Arenes Exercise AnswersDocument18 pagesOC04 Arenes Exercise Answersjavierheng314No ratings yet
- Neet Halo PaperDocument4 pagesNeet Halo PaperYASH KUMARNo ratings yet
- CL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDocument12 pagesCL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDivya KhandelwalNo ratings yet
- Class XII Haloalkanes, Alcohol, Phenol & EtherDocument4 pagesClass XII Haloalkanes, Alcohol, Phenol & EtherGourango NayakNo ratings yet
- M-Caps-34: Chemistry: NEET & AIIMS 2018-19Document5 pagesM-Caps-34: Chemistry: NEET & AIIMS 2018-19Vishal SinghNo ratings yet
- Alcohols and PhenolsDocument9 pagesAlcohols and Phenolsdivya divyaNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Document36 pagesCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarNo ratings yet
- Carboxylic Acid CPPDocument24 pagesCarboxylic Acid CPPGulshan kumarNo ratings yet
- Xicbse Che Haloalkanes Haloarenes 2 QPDocument3 pagesXicbse Che Haloalkanes Haloarenes 2 QPtanishkakannan3253No ratings yet
- Ncert Chemistry Xii 11Document36 pagesNcert Chemistry Xii 11Sunil Mattoo100% (1)
- Đề Cương Học Phần Hoá Hữu Cơ Lớp D2022Document17 pagesĐề Cương Học Phần Hoá Hữu Cơ Lớp D2022Cảnh NguyễnNo ratings yet
- Organic Questions 1Document3 pagesOrganic Questions 1SABARI SRINIVAS ANo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Alkyl HalidesDocument15 pagesAlkyl HalidesDjdj DjdjNo ratings yet
- Haloalkanes and HaloarenesDocument1 pageHaloalkanes and HaloarenesSneha Yadav0% (1)
- JEE Advanced 2013 SyllabusDocument8 pagesJEE Advanced 2013 SyllabusPragnesh ParekhNo ratings yet
- CBQ Haloalkanes and HaloarenesDocument20 pagesCBQ Haloalkanes and HaloarenesAnish dekaNo ratings yet
- Revised Xii Study Cfic Ms 2024-2025 Jee Micro Schedules 04-04-2024Document4 pagesRevised Xii Study Cfic Ms 2024-2025 Jee Micro Schedules 04-04-2024nawec98190No ratings yet
- Haloalkanes and HaloarenesDocument21 pagesHaloalkanes and HaloarenesRashmiNo ratings yet
- 2018SU B.SC Chemistry SyllabusDocument22 pages2018SU B.SC Chemistry Syllabussachin81185No ratings yet
- Chem. Imp. QuestionsDocument16 pagesChem. Imp. QuestionsHimanshu NikhurpaNo ratings yet
- ReasoningDocument4 pagesReasoningAayush MishraNo ratings yet
- Haloalkanes and Haloarenes Notes GoodDocument21 pagesHaloalkanes and Haloarenes Notes GoodAnitesh DharamNo ratings yet
- Haloalkane and HaloareneDocument8 pagesHaloalkane and HaloareneSaransh KumarNo ratings yet
- ORGANIC CHEMISTRY Class NotesDocument19 pagesORGANIC CHEMISTRY Class NotesWolam guyNo ratings yet
- Case Study Ques. of Haloalkanes and HaloarenesDocument3 pagesCase Study Ques. of Haloalkanes and HaloarenesAryan Sharma100% (1)
- Haloalkanes and Haloarenes-Anil-hssliveDocument3 pagesHaloalkanes and Haloarenes-Anil-hssliveNikhil MathewNo ratings yet
- Organic Chemistry Chapter Wise Previous Year Question PDFDocument13 pagesOrganic Chemistry Chapter Wise Previous Year Question PDFVishal SNo ratings yet
- NCERT Exemplar Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes PDFDocument39 pagesNCERT Exemplar Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes PDFSanjana SanjayNo ratings yet
- 10 Haloalkanes and Haloarenes 1 MK QuestionsDocument42 pages10 Haloalkanes and Haloarenes 1 MK QuestionsPriyanks RoutNo ratings yet
- Unit 10-Haloalkanes and HaloarenesDocument40 pagesUnit 10-Haloalkanes and HaloarenesHazur SahibNo ratings yet
- Haloalkanes and HaloarenesDocument35 pagesHaloalkanes and HaloarenesStarsNo ratings yet
- Ebook Chemistry Module V Organic Chemistry Ii For Iit Jee Main and Advanced Rajesh Agarwal Mcgraw Hill Education PDF Full Chapter PDFDocument68 pagesEbook Chemistry Module V Organic Chemistry Ii For Iit Jee Main and Advanced Rajesh Agarwal Mcgraw Hill Education PDF Full Chapter PDFmable.connors621100% (38)
- 1-CHSE 2024-25 XII Question Bank Chemistry Haloalkane and HaloarenesDocument14 pages1-CHSE 2024-25 XII Question Bank Chemistry Haloalkane and HaloarenesPapun BarikNo ratings yet
- Haloalkanes and HaloarenesDocument9 pagesHaloalkanes and HaloarenesManish kumarNo ratings yet
- NCERT Important Name Reactions For RevisionDocument34 pagesNCERT Important Name Reactions For Revisionyimisa2927No ratings yet
- The Amine-Catalysed Suzuki-Miyaura-type Coupling of Aryl Halides and Arylboronic AcidsDocument8 pagesThe Amine-Catalysed Suzuki-Miyaura-type Coupling of Aryl Halides and Arylboronic AcidsDinh Dong DOANNo ratings yet
- Haloalkanes and HaloarenesDocument26 pagesHaloalkanes and Haloarenesrajputrishi1982100% (1)
- Suzuki CouplingDocument63 pagesSuzuki CouplingVirendra RautNo ratings yet
- Important Chemical Reactions For Class 12 Chemistry With MechanismDocument13 pagesImportant Chemical Reactions For Class 12 Chemistry With Mechanismtejaschaturvedi098No ratings yet
- Haloalkanes and Haloarenes PDFDocument3 pagesHaloalkanes and Haloarenes PDFarya sonarNo ratings yet
- Assignment Haloalkanes and HaloarenesDocument3 pagesAssignment Haloalkanes and HaloarenesAayush RastogiNo ratings yet
- Exp.6-Alkyl and Aryl HalidesDocument16 pagesExp.6-Alkyl and Aryl HalideszazoNo ratings yet