Professional Documents
Culture Documents
straus1982_Acyclovir for chronic mucocutaneous HSV infection in immunocompromised px
straus1982_Acyclovir for chronic mucocutaneous HSV infection in immunocompromised px
Uploaded by
nisrinanfitriaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
straus1982_Acyclovir for chronic mucocutaneous HSV infection in immunocompromised px
straus1982_Acyclovir for chronic mucocutaneous HSV infection in immunocompromised px
Uploaded by
nisrinanfitriaCopyright:
Available Formats
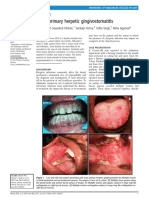
Acyclovir for Chronic Mucocutaneous Herpes Simplex Virus Infection in
Immunosuppressed Patients
STEPHEN E. STRAUS, M.D.; HOLLY A. SMITH, B.S.; CHAIM BRICKMAN, M.D.; PAULO de MIRANDA,
Ph.D.; COLIN McLAREN, Ph.D.; and RONALD E. KEENEY, M.D.; Bethesda, Maryland; and Research
Triangle Park, North Carolina
Over 25 episodes of severe chronic and recurrent herpes simplex virus infection was recorded ( 1 1 ) . Virus typing
mucocutaneous herpes simplex virus infections in five was done by a direct immunofluorescence assay ( 1 2 ) .
immunodeficient patients were successfully treated with Acyclovir capsules, 200 mg, and acyclovir sodium salt for
intravenous or oral acyclovir treatment. Acyclovir was intravenous administration were provided by Burroughs Well-
shown to inhibit viral shedding rapidly, to be well come Co. Acyclovir drug levels were measured by radioimmu-
tolerated, and to permit the complete healing of lesions. noassay ( 1 3 ) . The sensitivity of herpes simplex virus isolates to
As expected, a course of acyclovir did not prevent later inhibition by acyclovir was measured in a quantitative cyto-
recurrences of the herpes virus infections. However, pathic effect-reduction assay in microtiter plates using serial
symptomatic recurrences were successfully suppressed twofold dilutions of acyclovir. Vero cells were challenged with
during long (up to 65-day) courses of oral acyclovir. approximately 30 infectious units of the test virus, and the cyto-
pathic effect at 72 h was measured by uptake of neutral red dye
( 1 4 ) . The 50% infective dose values measured in this assay are
S O M E PATIENTS with defective cell-mediated immunity approximately 10-fold higher that those obtained in a standard
have primary or recurrent mucocutaneous herpes sim- plaque reduction assay (McLaren C. Unpublished observa-
plex virus infections that are more severe and longer last- tions).
ing than the corresponding infections in immunologically
competent hosts (1-5). Recently, intravenous acyclovir Case Reports
has been shown to be the first antiherpetic agent capable PATIENT 1
of decreasing the duration and severity of mucocutaneous This 3-year-old white boy was first referred to the N I H in
herpes simplex virus infections in compromised patients September 1979 for evaluation of chronic mucocutaneous le-
(6-9). Moreover, herpes simplex virus infections after sions and a suspected immune defect that later proved to be
bone marrow transplantation can be prevented during associated with nucleoside phosphorylase deficiency (Winkel-
stein J. Personal communication). At age 2 years, he had had a
treatment with prophylactic intravenous acyclovir ( 1 0 ) .
severe bout of acute encephalitis of undetermined cause that
We report clinical, virologic, and pharmacologic obser- had resulted in significant chronic neurologic impairment. The
vations of over 25 episodes of chronic and recurrent her- encephalitis was followed by the appearance for the first time of
pes simplex virus infections in five immunodeficient pa- vesicular and then chronic ulcerative lesions of the mouth and
tients. Data are presented that indicate repeated treat- face, which spontaneously resolved after a few weeks but re-
curred in July 1979. The lesions progressed prompting his refer-
ments of these infections with intravenous or oral acyclo- ral to the N I H Clinical Center.
vir are effective and well tolerated. Moreover, prolonged When first examined, large ulcerative lesions involved the
or individually tailored regimens of oral acyclovir seem right cheek, lips, tongue, and the crease of the neck. Viral cul-
to be capable of preventing expected clinical recurrences. tures yielded herpes simplex virus type 1. He was admitted to a
National Institute of Allergy and Infectious Diseases ( N I A I D )
collaborative adenine arabinoside (vidarabine)-placebo cross-
Methods over trial (randomly ordered sequential weeks of vidarabine, 10
All patients (Table 1) were evaluated and treated at the Na- mg/kg body weight d intravenously, or placebo infusions). The
tional Institutes of Health (NIH) Clinical Center after they clinical course and titers of viral shedding suggested little re-
gave fully informed consent. Inpatients with mucocutaneous le- sponse to that treatment (Figure 1). An open trial of vidarabine
sions were interviewed and examined daily; the severity of pain, at 20 mg/kg body weight • d was attempted with drying and loss
the stages, and the relative sizes of lesions were recorded. Le- of crusts from cheek lesions but progression of nose, mouth,
sions were considered to have healed when symptoms and signs and neck lesions (Figure 2 ) . After more than 4 months of pro-
of inflammation had resolved; complete reepithelialization was gressive lesions, acyclovir, 5 mg/kg body weight, was adminis-
not required. tered intravenously every 8 h for 5 days. Quantitative viral ti-
Virus cultures were done before and frequently during thera- ters and daily observation of lesions showed a prompt and dra-
py. Freshly aspirated vesicle fluid or lesion swabs were trans- matic improvement (Figures 1 and 2 ) . By the third day of
ported to the virology laboratory in veal infusion broth on ice treatment the lesions were clearly drier. Healing, but for residu-
and inoculated within 24 h into roller tubes, some containing al erythema, was complete in about 2 weeks. Subsequent cul-
monolayers of human diploid fibroblasts (WI-38) and others tures and examinations indicated a clinical and virologic recur-
containing human embryonic kidney cells. Quantitative titra- rence about 3 weeks after the acyclovir course was completed.
tions of virus shedding were done by inoculating serial 10-fold Oral, labial, and facial lesions progressed steadily, but retreat-
dilutions of the original specimens into WI-38 culture tubes, ment was postponed because other severe medical complica-
and were calculated as 50% tissue culture infective dose units tions developed, including Pneumocystis carinii and Legionella
per 0.2 mL. The appearance of typical cytopathologic effects of pneumophila pneumonias, from which he died.
PATIENT 2
• From the Laboratory of Clinical Investigation, National Institute of Allergy
and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; and The early history and responses to treatment of multiple her-
Burroughs Wellcome Co.; Research Triangle Park, North Carolina. pesvirus infections in this 67-year-old black woman have been
270 Annals of Internal Medicine. 1982;96:270-277.
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
Table 1 . Immunodeficient Patients Treated with Acyclovir
Patient Age Virus Type Location of Lesions Underlying Disorder
yrs
1 3 1 Face, neck, mouth Nucleoside phosphorylase deficiency
2 67 2 Vulva, perineum, buttocks Common variable immunodeficiency
3 27 2 Vulva, perineum, buttocks Common variable immunodeficiency
4 38 2 Face, hands, groin, penis, legs Common variable immunodeficiency
5 35 2 Buttocks, penis Acquired combined immunodeficiency
reported elsewhere ( 7 ) . Early in 1979 she developed Pneumo- frequently achieved. Further, we found that 3-day cycles of acy-
cystis pneumonitis, chronic perineal and vulvar herpes simplex clovir treatment, 200 mg by mouth every 4 h while awake (five
virus type 2 infection, and chronic disseminated varicella zoster times daily), alternated with 5-day cycles off the drug interrupt-
virus infection that failed to respond to intravenous vidarabine ed asymptomatic shedding and delayed the appearance of the
administered at another institution. By June 1979 she developed next clinical recurrence. Subsequent cycles of oral acyclovir giv-
cytomegalovirus pneumonia in addition to her other ongoing en three times daily for 2 consecutive days each week initially
herpesvirus infections. The pneumonia and skin lesions pro- appeared to be suppressive but ultimately permitted break-
gressed but resolved promptly after treatment was started with through of a clinial recurrence.
intravenous acyclovir, 5 mg/kg body weight every 8 h for 5 Her most recent preventive treatment (course 21) followed a
days. Approximately 2 weeks later and again several times dur- standard 5-day treatment (200 mg by mouth 5 times daily) and
ing the autumn of 1979, she had brief recurrences of vesicular consisted of 30 days of oral acyclovir at four capsules daily
eruptions involving the buttocks, perineum, and vulva. followed immediately by 30 days of three capsules daily. This
In November 1979, the perineal herpes simplex virus lesions regimen was well tolerated and seemed to suppress asympto-
reappeared and were soon followed by a second episode of dis- matic shedding and the appearance of lesions until several days
seminated zoster and recurrent pneumonia, possibly due to cy- after its completion.
tomegalovirus. Treatment at another hospital with vidarabine
and trimethoprim-sulfamethoxazole was unsuccessful. In mid-
December she was transferred back to the N I H where a second
course of acyclovir, 7.5 mg/kg body weight intravenously every
8 h for 5 days, was associated with prompt clinical resolution of
pneumonia and the cutaneous herpes simplex virus infections
(Figure 1). Since then, she has had chronic fungal osteomyeli-
tis, diffuse histiocytic lymphoma of the lung, and intermittent
self-limited episodes of herpes simplex virus involving the peri-
neum and buttocks.
PATIENT 3
This 27-year-old white woman had a long history of common
variable immunodeficiency complicated by recurrent severe
bacterial infections and numerous cutaneous papillomata. In
mid-April 1980 she had genital herpes for the first time. Over a
period of 6 weeks the infection progressed to ulcerate much of
the vulva and perineum, prostrated her, and necessitated blad-
der catheterization for urinary retention as well as administra-
tion of high doses of analgesics for severe pain. As with Patient
1, she was randomized to receive consecutive 1-week courses of
vidarabine or placebo infusions (Figures 1 and 3). Because she
failed to respond to that treatment, acyclovir, 250 m g / m 2 body
surface area, was intravenously administered every 8 h for 5
days. She responded promptly with inhibition of virus shedding
and improvement in symptoms so that no analgesia was needed
by the fifth day. The lesions were noticeably drier by day 3 and
completely healed in 2 weeks.
Since that first episode, she has had numerous clinically and
virologically documented recurrences of genital herpes (Figure
4); she repeatedly showed that her lesions would become very
large, exceedingly painful, and would fail to improve spontane-
ously without acyclovir treatment. By both clinical and virolog-
ic criteria, she promptly responded to four courses of intrave-
nous therapy and many courses of oral therapy (200 mg acyclo-
vir every 4 h while awake). The interval between completion of
one course of therapy and the next clinical recurrence estab-
lished a regular pattern of only 6 to 10 days. Moreover, asymp-
tomatic shedding was frequent and could begin as soon as 4 to 5
days after the acyclovir treatment was stopped. The interval to
the next recurrence was not significantly increased by reinstitut- Figure 1 . Responses of four patients to antiviral therapy with vidar-
abine (ARA) and placebo (PLC) and intravenous acyclovir (ACV) or
ing intravenous treatment (course 7 ) , or by 28- or 65-day oral acyclovir (fourth treatment of Patient 3). A. Patient 1 . B. Pa-
courses of oral acyclovir (courses 12 and 21). tient 2. C. Patient 3. D. Patient 5. The shaded areas show the
The absence of herpes lesions during long courses of acyclo- relative extent of lesions and symptoms; the height of the shading
vir treatment suggested that, as in the study of Saral and associ- reflects the severity of pain, the size of lesions, or both; solid black
ates (10), clinical recurrences of herpes simplex virus infections bars indicate the quantity of virus recovered; + = a positive (un-
could be suppressed as long as inhibitory acyclovir levels were quantitated) culture; — = a negative culture.
Straus et al. • Acyclovir for Herpes 271
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
In early 1981 a series of vesicular eruptions on the face and
groin were followed clinically, proved to be caused by herpes
simplex virus type 2, and then successfully treated with intrave-
nous acyclovir, 250 m g / m 2 body surface area every 8 h for 5
days (Figure 5). After that treatment, he was totally free of
lesions for nearly 2 weeks, the first time in over 2 years.
After three more episodes of overlapping recurrences at dif-
ferent sites, he was readmitted and treated successfully with
oral acyclovir, 200 mg every 4 h while awake. After a second
course of oral acyclovir, he was placed on an expectant, inter-
mittent suppressive regimen of oral acyclovir similar to that
used for Patient 3. He remained clinically free of lesions for
about 2 weeks after his last dose of acyclovir. Recurrent groin,
ear, and buttock lesions appeared and were successfully treated
with a 5-day course of acyclovir, 200 mg every 4 h while awake.
Subsequent recurrences have been suppressed by treatment
with 30 days of capsules given 4 times daily followed by 30 days
of three capsules a day (Figure 5). While at home during the
long suppressive regimen, he had two brief episodes of erythe-
ma and pain in the groin and chin area. The symptons lasted as
long as 2 days and resolved spontaneously. Cultures were not
obtained.
PATIENT 5
A 35-year-old male homosexual developed a syndrome that
has been recognized only recently in that population (15). In
the winter of 1980-1981 he had had several episodes of rather
mild genital lesions. In March 1981, he became severely ill,
persistently febrile, and began to lose weight rapidly. Between
March and May of 1981, Pneumocystis pneumonia, Candida
esophagitis, Mycobacterium avium-intracellulare infection,
hepatitis, and rising cytomegalovirus serologic titers were
found. In April an enlarging ulcerative lesion was noted in the
crease of the buttocks below the sacrum. A biopsy showed in-
tranuclear inclusions. In May, virus cultures recovered herpes
simplex virus type 2 from the lesions. The patient was trans-
ferred to the NIH Clinical Center for further immunologic eval-
uation.
He was found to be anergic, severely lymphopenic, and de-
void of all lymphocyte functions as assessed in vitro. Cultures of
urine, throat, blood, and bone marrow grew cytomegalovirus.
Retinoscopy showed a progressive acute retinitis. The sacral
ulcer remained culture positive for herpes simplex virus and
enlarged to encompass an area of about 25 cm 2 . Acyclovir infu-
sion was begun at a high dose of 500 m g / m 2 body surface area
intravenously every 8 h for 7 days with the hope of possibly
interrupting the cytomegalovirus infection as well as the herpes
simplex virus infection. The herpes simplex virus infection
cleared rapidly from the skin, and the ulceration healed within
10 to 14 days. New perianal lesions were noted about 3 weeks
after his last dose of acyclovir. Blood cultures positive for cyto-
megalovirus remained positive during acyclovir treatment.
The retinitis progressed further. Vidarabine, 15 mg/kg body
weight • d, was administered for 7 days with minimal effect upon
Figure 2. Results of antiviral treatment of Patient 1. Photographs the perineal lesions, but progression of retinitis appeared to halt
were taken (A) 1 day before the vidarabine and placebo treatment; transiently. He was retreated with acyclovir 500 m g / m 2 body
(B) 4 days after completing the double-dose open (second) vidara- surface area for 7 days in mid-September with rapid clearing of
bine (ARA-A) trial; and ( Q 3 days after completing acyclovir (ACV) his perianal lesions, but blood cultures remained positive for
treatment. Although the cheek lesions lost their crusts during the cytomegalovirus. By mid-October 1981 the perianal lesions had
second vidarabine trial, the mouth and neck lesions (not shown) reappeared and enlarged to generate a single ulcer of approxi-
were clinically unchanged, and the nasal lesion was worse. matley 100 cm 2 in area. This lesion benefited from treatment
PATIENT 4
with oral acyclovir but had not resolved at the time of the
patient's death on 28 October.
A 38-year-old white man with common variable immunodefi-
ciency developed severe primary genital herpes complicated by
culture-proven herpes simplex virus type 2 meningoencephalitis
in late 1978. The lesions of that primary episode gradually re- Results
solved spontaneously in 6 to 8 weeks. Over the next 2 years, he VIROLOGIC S T U D I E S
had near continuous lesions appearing on the penis, groin, but- Quantitative viral cultures were d o n e during 12 epi-
tocks, thighs, legs, hands, chin, and ears. The lesions would last
2 to 4 weeks and resolve spontaneously, but new lesions would sodes of recurrent herpes simplex virus infections in four
develop elsewhere before the crusts of the earlier lesions had patients ( F i g u r e 1 ) . Eight episodes were treated; t w o
fallen off. with randomly ordered w e e k - l o n g trials of vidarabine al-
272 March 1982 • Annals of Internal Medicine • Volume 96 • Number 3
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
Figure 3. Results of antiviral treatment of Patient 3. Photographs were taken (A) 1 day before vidarabine and placebo trial; (B) 1 day before
acyclovir treatment after completion of vidarabine (ARA-A) trial; and (C) 3 days after completion of acyclovir (ACV) treatment. Ulcerative
lesions progressed markedly during the vidarabine and placebo trial.
ternating with placebo infusions, five with intravenous PHARMACOLGY
acyclovir, and one with oral acyclovir. The data show Serum, urine, and stool specimens were obtained from
that acyclovir treatment was associated with a prompt Patients 1, 2, 3, and 4 and analyzed for acyclovir content
cessation of viral shedding and was followed in each case by radioimmunoassay. The data permit a number of ob-
by complete healing of lesions. Figures 2 and 3 illustrate servations on the metabolic fate and pharmacokinetics of
some of these excellent clinical responses. Figures 4 and 5 acyclovir in these patients. All patients had normal renal
depict the clinical and qualitative virologic observations function.
in Patients 3 and 4 before, during, and after repeated A total of 59 timed serum specimens, 35 24-h urine
successful therapeutic and suppressive trials. collections, and three 24-h stool collections were obtained
Figure 4. Results of oral acyclovir treatment regimens for Patient 3. Recurrent herpes simplex virus infection episodes five to 16 were
successfully treated. All were treated {dark boxes) with acyclovir, 2 0 0 mg five times daily, except course 6 that used 4 0 0 mg doses, and
course 7 that was administered intravenously with doses of 5 0 0 m g / m 2 body surface area. Intermittent short courses of preventive
treatment lasted 3 days (May) or 2 days (June, July) at dosages of five or three capsules daily, respectively. Long courses used five, four, or
three capsules daily (March to April, July to August, August to September, respectively). The shaded areas show the relative extent of
lesions and symptoms; the height of the shading reflects the severity of pain, the size of lesions, or both; solid black bars indicate acyclovir
administration; + = a positive (unquantitated) culture; — = a negative culture.
Straus eta/. • Acyclovir for Herpes 2 7 3
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
Figure 5. Results of acyclovir treat-
ment of Patient 4 . Intravenous
(first trial) and oral (all subsequent
trials) acyclovir treatments were
successfully used to terminate five
culture-proven episodes of recur-
rent herpesvirus infection. The
shaded areas show the relative ex-
tent of lesions and symptoms; the
height of the shading reflects the
severity of pain, the size of lesions,
or both; solid black bars indicate
acyclovir administration; + = a
positive (unquantitated) culture;
— = a negative culture.
during and after courses of intravenous acyclovir at dos- changed in the stool specimens analyzed.
ages of 5 mg/kg body weight, 7.5 mg/kg, and 250 m g / m 2
body surface area, every 8 h infused over 30 min. Peak TOXICOLOGIC S T U D I E S
serum levels of acyclovir (15 min after infusion) ranged The following side effects were found during acyclovir
from 40.9 to 60.4 jmmol, and from 62.6 to 90.1 jmmol after treatments. Patients 4 and 5 had cutaneous irritation and
doses of 5 mg/kg body weight and 7.5 mg/kg, respective- erythema from the drug, which was infused through im-
ly. Trough levels (5 to 10 min before dose) ranged from properly situated intravenous catheters. Patient 3 devel-
3.8 to 12.0 u,mol, and from 9.3 to 17.2 jumol for these oped two, day-long episodes of nausea and three bouts of
dosages, respectively. severe diarrhea while on oral acyclovir. These appeared
Kinetic studies of serum drug concentrations were to be due to bacteriologically proven recurrences of Sal-
done at the dosages of 5.0 mg/kg body weight (Patient 1, monella enteritis that she had had previously. Patient 1
data not shown) and 7.5 mg/kg body weight (Patient 2, developed a tremor 1 week after acyclovir therapy. The
Figure 6 A ) . After the drug infusion was completed, the analysis of that complication is confounded because he
rate of fall in serum acyclovir levels was rapid at first but had recently completed two courses of vidarabine, a drug
then slowed considerably, in accord with a biexponential known to be associated with tremor, and because his neu-
model of drug distribution described earlier for acyclovir rologic status before treatment was severely abnormal.
(16). Over a 5-day course of two infusions daily, there Every 3 to 5 days during all of the acyclovir treatment
was only a slight tendency toward gradual accumulation regimens and every 1 to 2 weeks during the suppressive
of drug. Trough drug levels greater than those needed to regimens, the patients had full batteries of blood and
completely inhibit herpes simplex virus replication were urine tests for evidence of drug-related toxicity. Patient 2
present throughout the 8-h period between doses. developed a transient twofold rise in serum transaminase
Further detailed analyses of the serum levels and drug levels during her first course of intravenous acyclovir.
excretion were done in two patients treated with intrave- The level started decreasing toward normal while the pa-
nous acyclovir and later with oral acyclovir (Table 2 ) . tient was on acyclovir, and was normal within 1 week
The data indicate that after intravenous acyclovir admin- after completion of therapy. Patient 5 developed a tran-
istration about two thirds of the total dose appears un- sient leukopenia with a fall in his total leukocyte count
changed in the urine (Figure 6B). In first reporting this from 8000 to 2900 cells/mm 3 during his first intravenous
finding, de Miranda and associates (16) observed that acyclovir treatment. Other than these findings, no abnor-
renal acyclovir clearance involves both glomerular filtra- malities of complete blood count, differential count, reti-
tion and tubular secretion. Little of the drug is excreted culocyte count, SMA-18, or urinalysis were found.
in the stool.
After oral acyclovir administration, drug absorption is D R U G SENSITIVITY S T U D I E S
slow and variable, peaking between 1 and 4 h (de Miran- Herpes simplex virus isolates recovered before, during,
da P. Unpublished studies). All our data were collected and after treatment were tested for their sensitivity to
60 min after drug administration, and, therefore, proba- acyclovir (Table 3). Although some differences in drug
bly underestimated the actual peak serum levels. None- sensitivity occurred from specimen to specimen for each
theless, rather modest yet inhibitory levels were achieved patient, this finding was within the range of experimental
and sustained for at least as long as the interval between variation. In addition, the herpes simplex virus isolates
doses. Gastrointestinal absorption is rather poor with to- from the two specimens of Patient 3 with the greatest
tal bioavailability estimated to be in the range of 15% to disparity in 50% infective dose (specimens 2 and 4) were
30% (Blum R, de Miranda P. Unpublished studies). shown to express comparable levels of thymidine kinase
About 10% to 15% of the drug was excreted unchanged activity (results not shown) (18). Thus, we believe that
in the urine, whereas 15% to 25% was excreted un- the herpes simplex virus that reactivated after treatment
2 7 4 March 1982 • Annals of Internal Medicine • Volume 96 • Number 3
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
Figure 6. Pharmacokinetics and urinary excretion of acyclovir after intravenous administration. A. Patient 2. Serum drug levels after the
first three doses of intravenous acyclovir (ACV), 7.5 m g / k g body weight-8 h. B. Patient 3. Percent of daily acyclovir dose recovered in -24-h
urine collections during 7 consecutive days after the initiation of a 5-day course of intravenous acyclovir, 2 5 0 m g / m 2 body surface area-8
h. Data are expressed as ± 1 SD.
was completed did not differ greatly in their sensitivity to to improve markedly during controlled trials of vidara-
acyclovir from the isolates recovered before antiviral bine and placebo. Despite this, however, the objective
therapy. and subjective data are sufficiently compelling and consis-
tent with the data of other recent human trials of acyclo-
Discussion vir therapy (6-10) as to be entirely reasonable.
In terms of the extent, chronicity, and frequency of The biochemical mechanism that underlies the ability
recurrence, the lesions in the five patients reported here of acyclovir to inhibit herpes simplex virus replication is
represent the severe end of the spectrum of mucocutane- known (17, 18). The drug is converted to the monophos-
ous herpes simplex virus infections that are found in the phate form by the virally coded pyrimidine deoxynucleo-
compromised host. As such, it is important to recognize side (thymidine) kinase, a reaction that proceeds only
that the challenge to successful therapy was greater than in slightly in uninfected cells. Acyclovir monophosphate is
most persons. We have shown that challenging infections subsequently converted by cellular enzymes to the di-
such as these can be effectively treated with intravenous or and ultimately the triphosphate form that selectively in-
oral acyclovir. All courses of treatment were associated hibits herpes simplex virus-encoded D N A polymerase.
with a rapid suppression of virus shedding and permitted The concentration of acyclovir that inhibits cell D N A
complete healing of lesions. Some of the successfully treat- synthesis in uninfected cells is at least several hundred
ed infections had been present for over 4 months and had times higher than the concentration needed to inhibit
proven to be refractory to vidarabine. In addition, we have herpes simplex virus D N A synthesis to an equivalent de-
shown that expected clinical recurrences can be prevented gree. Acyclovir has the greatest activity against herpes
as long as acyclovir treatment is continued. simplex virus type 1 with a mean 50% plaque-reducing
It is possible that the clinical responses in the five pa- inhibitory dose between 0.1 and 0.5 jumol and slightly
tients were fortuitous and not related to acyclovir admin- less activity against herpes simplex virus type 2 (0.5 to 2
istration. These patients were not treated with acyclovir jimol) (19, 20). These differences should not be clinically
in blinded, controlled trials although two patients failed significant.
Table 2. Distribution and Excretion of Acyclovir During Intravenous <3r Oral Therapy*
Doset Patient Serum Levels* Daily Dose Recovered in
Peak Trough 24-h Urine 24-h Stool
jumoJ %
Intravenous
250 mg/m2 . 8 h 3 29 A ± 10.4 (7) 2.7 ± 1.3 ( 9 ) 64.4 ± 1 5 . 0 ( 1 1 ) 0.7 ± 0.6 ( 2 )
250 mg/m2 . 8 h 4 41.0 ± 5.0 (2) 7.5 ± 0.2 (2) 66.0 ± 15.8 (3) 0.0(1)
Oral
200 m g / 4 h 3 2.3 ± 0.9 (7) 2.3 ± 0.9 (7) 12.3 ± 3.9 (8) 19.5 ± 6.7 ( 5 )
400 m g / 4 h 3 3.5 ± 1.4 ( 4 ) 4.5(1) 11.8 ± 1.1 (2) Not done
* All data are expressed as means ± 1 standard deviation for the number of independent measurements shown in the parentheses.
t Intravenous dose is expressed in mg acyclovir / m 2 body surface area.
% Intravenous courses specimens for peak serum levels were taken 15 min after the end of an infusion whereas trough serum level samples were collected 5 to 10 min
before starting an infusion other than that for the first dose. For oral courses, "peak" levels were estimated with samples taken at 60 min after the dose. "Trough" levels
were taken 4 h after dose administration (5 to 10 min before subsequent doses).
Straus etaf. • Acyclovir for Herpes 2 7 5
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
Table 3. Sensitivity of Herpes Simplex Virus Isolates to Acyclovir
Patient Test Date Identity 50% Infective
Number Dose*
jug/mL
1 1 25 September 1979 Before treatment 1 0.20 ± 0.08
2 7 December 1979 After treatment 1 0.36 ± 0.06
2 1 30 May 1979 Before treatment 1 0.76 ± 0 . 1 7
2 7 July 1979 During treatment 1 1.29 ± 0 . 2 0
3 23 December 1979 After treatment 1 0.58 ± 0.20
3 1 27 May 1980 Before treatment 1 0.44 ± 0 . 1 1
2 1 August 1980 After treatment 1 0.23 ± 0.04
3 24 November 1980 After treatment 5 1.03 ± 0 . 0 9
4 27 January 1981 During treatment 9 1.59 ± 0 . 2 2
5 24 March 1981 After treatment 11 0.46 ± 0.07
6 4 May 1981 After treatment 12 0.41 ± 0.07
* The mean ± SE concentration of acyclovir needed to produce a 50% inhibition of herpes simplex virus as indicated by dye uptake. These results are approximately 10
times greater than those obtained by the plaque reduction method. For acyclovir, 1 fig/mL is approximately equal to 4.35 umol.
The pharmacologic studies of our patients indicate that viral thymidine kinase gene, one mechanism for develop-
their handling of acyclovir was comparable to that found ment of acyclovir resistance, are associated with a signifi-
previously ( 1 6 ) . With intravenous therapy, mean peak cant diminution or the elimination of the capacity of the
serum levels of 30 to 40 u-mol were achieved at 15 min virus to establish neural latency (23). Nonetheless, the
after infusion, and mean trough serum levels were 3 to 8 potential risk of emergence of clinically important acy-
jutmol. With oral therapy, mean peak and trough serum clovir-resistant herpes simplex virus exists not only for
drug levels were both about to 2 to 3 umol. Although the treated patient but also for potential contacts. For
oral acyclovir was relatively poorly absorbed from the persons who have frequent recurrences, chronic therapy
gastrointestinal tract, levels exceeding those required to may be preferable to intermittent expectant therapy.
inhibit the replication of herpes simplex virus types 1 or 2 Then the virus would not have the opportunity to repeat-
were readily achieved and sustained for several hours. edly reactivate and be exposed to suboptimal drug con-
There are risks associated with the acyclovir treat- centrations. On the other hand, we believe the develop-
ments used, particularly those given to Patients 3 and 4. ment and understanding of antiviral drugs and their po-
Repeated courses of acyclovir enhance the likelihood of tential toxicities has not yet grown sufficiently to permit
drug-related toxicity. It is noteworthy that the acyclovir us to recommend very long-term suppressive regimens.
treatment regimens for the patients presented here and A C K N O W L E D G M E N T S : The authors thank the Burroughs Wellcome
for the much larger number of patients reported else- Co. for providing the acyclovir; J. Owens, E. Keith, D. Page, S. Good, and
G. Hunter for technical help; P. Keller for doing the viral thymidine kinase
where (6, 8-10) were infrequently associated with toxici- assays; T. Creagh-Kirk for additional logistical support; K. Leighty for man-
ty. When apparent, the side effects were uniformly of a uscript preparation and editing; S. Bachrach, R.N., and many Clinical Asso-
mild and reversible nature. ciates for assistance in patient care; and Drs. A. S. Fauci, A. Muchmore, and
T. Waldmann for patient referrals.
More importantly, though, repeated acyclovir treat-
ments would be expected to favor selection of drug-resis- • Requests for reprints should be addressed to Stephen E. Straus, M.D.;
Medical Virology Section, LCI, NIAID, Building 10, Room ll-N-113,
tant virus strains. Therefore, it is noteworthy that we NIH; Bethesda, M D 20205.
failed to find drug-resistance in our patients. Acyclovir-
resistant mutants of herpes simplex virus are readily gen- References
erated in the laboratory, and some resistant clinical iso- 1. NAHMAIS AJ, ROIZMAN B. Infection with herpes-simplex viruses 1 and
2. N Engl J Med. 1973;289:667-74, 719-25, 781-9.
lates have been recovered ( 2 1 ) . Theoretically, because 2. S T O N E WJ, S C O W D E N EB, S P A N N U T H CL, L O W R Y SP, A L F O R D RH.
most recurrences of herpes simplex virus infection repre- Atypical herpes simplex virus hominis type 2 infection in uremic pa-
tients receiving immunosuppressive therapy. Am J Med. 1977;63:511-6.
sent reactivation of the same latent viral genome ( 2 2 ) ,
3. PASS RF, W H I T L E Y RJ, W H E L C H E L J D , D I E T H E L M AG, R E Y N O L D S
drug resistance should not develop while adequate inhibi- DW, A L F O R D CA. Identification of patients with increased risk of infec-
tory levels of acyclovir are maintained. Clinically signifi- tion with herpes simplex virus after renal transplantation. J Infect Dis.
1979;140:487-92.
cant drug resistance may be possible, however, if latency 4. MEYERS, J D , FLOURNEY N, THOMAS ED. Infection with herpes sim-
had to be reestablished with each recurrence. Experience plex virus and cell-mediated immunity after marrow transplant.
with potent antiherpetic drugs like acyclovir indicates 5. O V E R A L L JC J R . Dermatologic diseases. In: G A L A S S O G, M E R I G A N
TC, BUCHANAN RA, eds. Antiviral Agents and Viral Diseases of Man.
that this may not occur routinely because recurrences New York; Raven Press; 1979:305-83.
develop despite rapid virus clearance. A more attractive 6. SELBY PJ, P O W L E S RC, JAMESON B, et al. Parenteral acyclovir therapy
hypothesis is that drug resistance in a person already har- for herpes simplex virus infections of man. Lancet. 1979;2:1267-70.
7. C U P P S TR, STRAUS SE, W A L D M A N N TA. Successful treatment with
boring herpes simplex virus may occur when latency is acyclovir of an immunodeficient patient infected simultaneously with
established in a new group of nerve cells by reactivated multiple herpes simplex viruses. Am J Med. 1981;70:882-6.
8. M I T C H E L L CD, G E N T R Y SR, BOEN JR, BEAN B, G R O U T H KE, B A L -
virus that mutated during exposure to subinhibitory lev- FOUR HH J R . Acyclovir therapy for mucocutaneous herpes simplex in-
els of acyclovir. Persons could endogenously reinfect fections in immune compromised patients. Lancet. 1981;2:1389-92.
themselves and potentially reactivate either of two clones 9. C H O U S, G A L L A G H E R JG, M E R I G A N TC. Controlled clinical trial of
intravenous acyclovir in heart-transplant patients with mucocutaneous
of virus, one sensitive to and one resistant to acyclovir. herpes simplex infections. Lancet. 1981;2:1392-4.
Fortunately, early studies suggest that mutations in the 10. SARAL R, B U R N S WH, LASKIN OL, SANTOS GW, L I E T M A N PS. Acy-
276 March 1982 • Annals of Internal Medicine • Volume 96 • Number 3
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
clovir prophylaxis of herpes-simplex-virus infections. A randomized, 18. F Y F E JA, K E L L E R PM, F U R M A N PA, M I L L E R RL, E L I O N GB. Thymi-
double-blind, controlled trial in bone-marrow-transplant recipients. TV dine kinase from herpes simplex virus phosphorylates the new antiviral
Engl J Med. 1981 ;305:63-7. compound, 9- (2-hydroxyethoxy methyl) guanine. / Biol Chem.
11. BLAIR JE, L E N N E T T E EH, T R U A N T JP. Manual of Clinical Microbiolo- 1978;253:8721-7.
gy. Baltimore: Williams and Wilkins; 1970. 19. SCHAEFFER HJ, B E A U C H A M P L, D E M I R A N D A P, E L I O N G B , B A U E R
12. N A H M I A S AJ, C H I A N G WT, D E L B U O N O I, D U F F E Y A. Typing of her- DJ, COLLINS P. 9-(2-Hydroxyethoxymethyl)guanine activity against vi-
pes simplex virus hominis strains by direct immunofluorescent tech- ruses of the herpes group. Nature. 1978;272:583-5.
nique. Proc Soc Exp Biol Med. 1969;132:386-90. 20. C R U M P A C K E R CS, SCHNIPPER LE, Z A I A JA, L E V I N MJ. Growth inhi-
13. Q U I N N RP, DE M I R A N D A P, G E R A L D L, G O O D SS. A sensitive radioim- bition by acycloguanosine of herpes simplex viruses isolated from hu-
munoassay for the antiviral agent BW 248 U [9-(2-hydroxyethoxyme- man infections. Antimicrob Agents Chemother. 1979;15:642-5.
thyl) guanine]. Anal Biochem. 1979;98:319-28. 21. SIBRACK CD, G U T M A N LT, W I L F E R T CM, M C L A R E N C, BARRY DW,
14. FINTER NB. Dye uptake methods for assessing viral cytopathogenicity Altered pathogenicity of acyclovir (ACV) resistant HSV from an im-
and their application to interferon assays. J Gen Virol. 1969;5:419-27. munodeficient child. Pediatr Res. 1981;15:621. Abstract.
15. C E N T E R FOR D I S E A S E C O N T R O L . Pneumocystis pneumonia—Los An- 22. B U C H A N A N TG, R O I Z M A N B, N A H M I A S AJ. Demonstration of exoge-
geles. 1981;30:250-2. nous genital reinfection with herpes simplex virus type 2 by restriction
16. D E M I R A N D A P, W H I T L E Y RJ, B L U M MR, et al. Acyclovir kinetics endonuclease fingerprinting of viral DNA. J Infect Dis. 1979; 140:295-
after intravenous infusion. Clin Pharm Ther. 1979;26:718-28. 304.
17. E L I O N GB, F U R M A N PA, F Y F E JA, D E M I R A N D A P, B E A U C H A M P L, 23. TENSER RB, M I L L E R RL, R A P P F. Trigeminal ganglion infection by
SCHAEFFER H. Selectivity of action of an antiherpetic agent, 9-(2-hy- thymidine kinase-negative mutants of herpes simplex virus. Science.
droxyethoxymethyl)guanine. Proc Natl Acad Sci (USA). 1977;74:5716- 1979;205:915-7.
20.
© 1982 American College of Physicians
Annals of Internal Medicine. 1982;96:277-280. 277
Downloaded From: http://annals.org/pdfaccess.ashx?url=/data/journals/aim/19581/ by a University of California San Diego User on 06/08/2017
You might also like
- Fatal Pulmonary Infection With Respiratory SyncytiDocument5 pagesFatal Pulmonary Infection With Respiratory Syncyti21701101046 Devi NavilaNo ratings yet
- Pi Is 1875957213001903Document5 pagesPi Is 1875957213001903eppy syafriNo ratings yet
- Disseminated Primary Herpes Simplex Virus Type 2 Infection in A 22-Year-Old MaleDocument3 pagesDisseminated Primary Herpes Simplex Virus Type 2 Infection in A 22-Year-Old MaleimanteguhbNo ratings yet
- Howard2019 PDFDocument3 pagesHoward2019 PDFNazihan Safitri AlkatiriNo ratings yet
- Eczema Herpeticum in An Infant - 2021 - JaipDocument2 pagesEczema Herpeticum in An Infant - 2021 - Jaiphazam2503No ratings yet
- Ramsay Hunt SyndromeDocument3 pagesRamsay Hunt SyndromepicassowaffleNo ratings yet
- Herpes Simplex Virus Laryngitis Presenting As Airway Obstruction: A Case Report and Literature ReviewDocument5 pagesHerpes Simplex Virus Laryngitis Presenting As Airway Obstruction: A Case Report and Literature ReviewEva PerdanaNo ratings yet
- Herpes Simplex Virus and Human Papillomavirus Coinfections in Hyperimmunoglobulin E Syndrome Presenting As A Conjunctival Mass LesionDocument5 pagesHerpes Simplex Virus and Human Papillomavirus Coinfections in Hyperimmunoglobulin E Syndrome Presenting As A Conjunctival Mass LesionWalid AissaNo ratings yet
- Chronic Virus Report of Three Cases Indolent Orofacial Herpes Simplex Infection in Chronic Leukemia: ADocument4 pagesChronic Virus Report of Three Cases Indolent Orofacial Herpes Simplex Infection in Chronic Leukemia: ANatalia RafaelNo ratings yet
- Rabella Et Al 2020 PDFDocument6 pagesRabella Et Al 2020 PDFmiftahus siddiqNo ratings yet
- Acute Necrotizing Sinusitis Caused by Staphylococcus Lugdunensis 2011Document3 pagesAcute Necrotizing Sinusitis Caused by Staphylococcus Lugdunensis 2011J Sebastian PeinadoNo ratings yet
- Nebulized Colistin in The Treatment of Pneumonia Due To Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas AeruginosaDocument4 pagesNebulized Colistin in The Treatment of Pneumonia Due To Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas AeruginosaPhan Tấn TàiNo ratings yet
- Jurnal Viro AccDocument6 pagesJurnal Viro AccMelisa FransiskaNo ratings yet
- 1 s2.0 S0213005X19302101Document3 pages1 s2.0 S0213005X19302101A MFNo ratings yet
- Acute Epiglottitis in The Immunocompromised Host: Case Report and Review of The LiteratureDocument5 pagesAcute Epiglottitis in The Immunocompromised Host: Case Report and Review of The Literaturerosandi sabollaNo ratings yet
- Dechlorane Plus and Related Compounds in Peregrine Falcon (Falco Peregrinus) Eggs From Canada and SpainDocument6 pagesDechlorane Plus and Related Compounds in Peregrine Falcon (Falco Peregrinus) Eggs From Canada and SpainlacosNo ratings yet
- Acute Appendicitis As A Complication of Varicella: Case ReportDocument3 pagesAcute Appendicitis As A Complication of Varicella: Case ReportAngel AdileNo ratings yet
- VaccineDocument7 pagesVaccineRong LiuNo ratings yet
- Single-Dose, Patient-Initiated Famciclovir: A Randomized, Double-Blind, Placebo-Controlled Trial For Episodic Treatment of Herpes LabialisDocument7 pagesSingle-Dose, Patient-Initiated Famciclovir: A Randomized, Double-Blind, Placebo-Controlled Trial For Episodic Treatment of Herpes LabialisnnNo ratings yet
- Noor2016 PDFDocument13 pagesNoor2016 PDFBilel DhaouadiNo ratings yet
- Virology ModuleDocument165 pagesVirology ModuleAhmed KerAtyNo ratings yet
- Admin A 10 1 168 Cd5e504Document5 pagesAdmin A 10 1 168 Cd5e504Bara Bagus RamandaNo ratings yet
- Ofy 038Document5 pagesOfy 038Rosandi AdamNo ratings yet
- 81 - Sinococulin A Bioactive Compound Has Anti Viral Activity Against DengueDocument13 pages81 - Sinococulin A Bioactive Compound Has Anti Viral Activity Against DenguesuhasmhaskeNo ratings yet
- Laporan KasusDocument6 pagesLaporan KasusNovia DamayantiNo ratings yet
- Jamadermatol 2020 0013Document2 pagesJamadermatol 2020 0013Mostafa SalahNo ratings yet
- 140794.3 20210324105625 CoveredDocument16 pages140794.3 20210324105625 CoveredBruno RamírezNo ratings yet
- Rare Presentation of Mycobacterium Avium-Intracellulare InfectionDocument3 pagesRare Presentation of Mycobacterium Avium-Intracellulare InfectionVenter CiprianNo ratings yet
- Acute Primary Herpetic Gingivostomatitis: Ravi Prakash Sasankoti Mohan, Sankalp Verma, Udita Singh, Neha AgarwalDocument3 pagesAcute Primary Herpetic Gingivostomatitis: Ravi Prakash Sasankoti Mohan, Sankalp Verma, Udita Singh, Neha AgarwalBrili AnenoNo ratings yet
- Chronic Herpes GenitalDocument3 pagesChronic Herpes GenitalYanna RizkiaNo ratings yet
- Epidemiology of Oral Candidiasis in HIV-Infected Patients: Colonization, Infection, Treatment, and Emergence of Fluconazole ResistanceDocument8 pagesEpidemiology of Oral Candidiasis in HIV-Infected Patients: Colonization, Infection, Treatment, and Emergence of Fluconazole ResistancePieter ReinaldoNo ratings yet
- Laryngeal TuberculosisDocument5 pagesLaryngeal TuberculosisastralmaniaNo ratings yet
- Answers To Continuing Medical Education QuestionsDocument2 pagesAnswers To Continuing Medical Education QuestionsKhalid AbdullahNo ratings yet
- Medically Important Viruses: The Methodical Manual For Medical StudentsDocument75 pagesMedically Important Viruses: The Methodical Manual For Medical Studentsdan ghaNo ratings yet
- Rabies VaccinesDocument2 pagesRabies VaccinesmadebagussnNo ratings yet
- Review 2 PDFDocument5 pagesReview 2 PDFJhair E. GodoyNo ratings yet
- A 65-Year-Old Man Came To The Emergency Department Via Ambulance - Relatives Accompanied The Patient and Described A 1-Day History of FeverDocument43 pagesA 65-Year-Old Man Came To The Emergency Department Via Ambulance - Relatives Accompanied The Patient and Described A 1-Day History of FeverandriopaNo ratings yet
- Valacyclovir in The Treatment of Herpes Simplex HeDocument11 pagesValacyclovir in The Treatment of Herpes Simplex HeGrnitrv 22No ratings yet
- AdenovirusDocument14 pagesAdenoviruszoomar muzammilNo ratings yet
- Sociedad Alemana Parte 1Document8 pagesSociedad Alemana Parte 1A Joel ZjNo ratings yet
- Cotterman2009 PDFDocument3 pagesCotterman2009 PDFAnonymous hxXpvZdZNo ratings yet
- Strongyloides Stercoralis Infection Causing Reversible Chronic Urticaria With Histologic Findings of Leukocytoclastic VasculitisDocument3 pagesStrongyloides Stercoralis Infection Causing Reversible Chronic Urticaria With Histologic Findings of Leukocytoclastic VasculitisGary Elvis TQNo ratings yet
- VirusDocument51 pagesVirusBatool SherbiniNo ratings yet
- Does Acyclovir Help Herpes Simplex Virus Cold Sores If Treatment Is Delayed (2004)Document3 pagesDoes Acyclovir Help Herpes Simplex Virus Cold Sores If Treatment Is Delayed (2004)Aron RonalNo ratings yet
- Scabies Norwegian Presenting As Erythroderma in HIV A Case ReportDocument4 pagesScabies Norwegian Presenting As Erythroderma in HIV A Case ReportfakhrunnisaNo ratings yet
- 1 s2.0 S2405469023001590 MainDocument3 pages1 s2.0 S2405469023001590 MainLeo TorresNo ratings yet
- What Treatment Orbital CellulitisDocument3 pagesWhat Treatment Orbital CellulitisDara Agusti MaulidyaNo ratings yet
- Hospital Pediatrics 2011 McCulloh 52 4Document5 pagesHospital Pediatrics 2011 McCulloh 52 4Denso Antonius LimNo ratings yet
- Herepes PDFDocument8 pagesHerepes PDFsuruthiNo ratings yet
- coinfeccion y superinfeccionDocument5 pagescoinfeccion y superinfeccionSEVILLA RAMIREZ JOSE MANUELNo ratings yet
- Splenectomy VaccinesDocument4 pagesSplenectomy VaccinesameliaNo ratings yet
- Treatmnt HerpesDocument8 pagesTreatmnt HerpesZakky FebrianNo ratings yet
- Elife 77345 v1Document48 pagesElife 77345 v1Afandi CharlesNo ratings yet
- Wang 2017 KKKDocument5 pagesWang 2017 KKKTais LineNo ratings yet
- Stevens-Johnson Syndrome Without Skin Lesions: Case ReportDocument4 pagesStevens-Johnson Syndrome Without Skin Lesions: Case ReportYona LadyventiniNo ratings yet
- Escholarship UC Item 9tv6p5jzDocument7 pagesEscholarship UC Item 9tv6p5jzgadangNo ratings yet
- Ophthalmia Neonatorum - 2020Document2 pagesOphthalmia Neonatorum - 2020CARLOS SANTIAGO PEREZ RODRIGUEZNo ratings yet
- Paralytic Poliomyelitis Caused by A Vaccine-Derived Polio Virus in An Antibody-Deficient Argentinean ChildDocument3 pagesParalytic Poliomyelitis Caused by A Vaccine-Derived Polio Virus in An Antibody-Deficient Argentinean Childxiongmao2389No ratings yet
- Aspergilloma of The Maxillary Sinus Complicating OACDocument3 pagesAspergilloma of The Maxillary Sinus Complicating OACabeer alrofaeyNo ratings yet
- Functional TestingDocument21 pagesFunctional TestingGogy MarkNo ratings yet
- Narrative Report For The Dengue Orientation ProgramDocument4 pagesNarrative Report For The Dengue Orientation ProgramDhenniz FlorezNo ratings yet
- Model Intervensi Hipertensi Di Kabupaten Lebak Provinsi BantenDocument8 pagesModel Intervensi Hipertensi Di Kabupaten Lebak Provinsi Bantenyanuar anazdiNo ratings yet
- MCQ Toxicology 2 PDFDocument16 pagesMCQ Toxicology 2 PDFMarwaFakhro50% (6)
- The BrainDocument2 pagesThe BrainAlejandroGonzagaNo ratings yet
- Handbook of Minerals As Nutritional Supplements PDFDocument254 pagesHandbook of Minerals As Nutritional Supplements PDFMelissa STanNo ratings yet
- (2018) Otake - ALTAIR StudyDocument5 pages(2018) Otake - ALTAIR StudyfikriafisNo ratings yet
- The Scale of Perceived Occupational StressDocument6 pagesThe Scale of Perceived Occupational StressPranay PandeyNo ratings yet
- Jurnal Massage For Infantile ColicDocument10 pagesJurnal Massage For Infantile ColicrambuubeNo ratings yet
- MPH Program in UP ManilaDocument2 pagesMPH Program in UP ManilaHannah Marquiala PellejoNo ratings yet
- How To Write A Good Conclusion For A Research PaperDocument131 pagesHow To Write A Good Conclusion For A Research PaperafodeiqlocvdppNo ratings yet
- Hollander and Berlin 2008 MeasuresDocument20 pagesHollander and Berlin 2008 MeasuresNISHANTHA KARUNARATHNANo ratings yet
- Health Belief Model UpdateDocument15 pagesHealth Belief Model Updatejust nomiNo ratings yet
- Leptospira and LeptospirosisDocument34 pagesLeptospira and LeptospirosisRegiane RibeiroNo ratings yet
- Ensuring A Patient's Right To Pastoral Care and Spiritual ServicesDocument6 pagesEnsuring A Patient's Right To Pastoral Care and Spiritual ServicesArwenn BeragoNo ratings yet
- I Contain MultitudesDocument8 pagesI Contain MultitudesIvan MontNo ratings yet
- Romeo and Juliet Death EssayDocument5 pagesRomeo and Juliet Death EssayiygcdknbfNo ratings yet
- Patofisiologi Group 5Document17 pagesPatofisiologi Group 5wiyadiNo ratings yet
- Hubungan Karakteristik Individu Dan Iklim Kerja Dengan Keluhan Msds Pada Pekerja Perakitan Mini Bus Di PT Mekar Armada Jaya MagelangDocument11 pagesHubungan Karakteristik Individu Dan Iklim Kerja Dengan Keluhan Msds Pada Pekerja Perakitan Mini Bus Di PT Mekar Armada Jaya MagelangDewi SiregarNo ratings yet
- Kaufmann Protocol GuidelinesDocument3 pagesKaufmann Protocol Guidelineszlautarevic-1No ratings yet
- Pregnancy With Previous LSCS: Vishal Final YearDocument21 pagesPregnancy With Previous LSCS: Vishal Final YearAdit RockNo ratings yet
- SkedDocument139 pagesSkedDaniel Edward Ricardo MalauNo ratings yet
- Florence Nightingale Theory: She Emphasizes Five Essential Point in Securing Health of HouseDocument2 pagesFlorence Nightingale Theory: She Emphasizes Five Essential Point in Securing Health of HouseAkshata BansodeNo ratings yet
- Prosthodontic Implant DrivenDocument7 pagesProsthodontic Implant DrivenbuccalNo ratings yet
- Rudolf Steiner On Viral Illness and EpidemicsDocument11 pagesRudolf Steiner On Viral Illness and EpidemicsnovalisNo ratings yet
- Dire Dawa University: College of Medicine and Health Science Department of MidwiferyDocument10 pagesDire Dawa University: College of Medicine and Health Science Department of MidwiferyYoseph KassaNo ratings yet
- North Luzon Philippines State College: Adal A Dekalidad, Dur-As Ti PanagbiagDocument1 pageNorth Luzon Philippines State College: Adal A Dekalidad, Dur-As Ti PanagbiagRandy Gasalao100% (1)
- Drug Study For Prednisone Case INPDocument2 pagesDrug Study For Prednisone Case INPChristina Barroga100% (4)
- Play Therapy Considerations and Applications Fir The PractitionerDocument6 pagesPlay Therapy Considerations and Applications Fir The PractitionerDiana IgnatNo ratings yet
- The Structure and Function of The Ear and Its Role in Hearing and Balance1Document4 pagesThe Structure and Function of The Ear and Its Role in Hearing and Balance1Khushbakht QureshiNo ratings yet