Professional Documents
Culture Documents
P3060760 Titrage conductimétrique, avec Cobra4-1
P3060760 Titrage conductimétrique, avec Cobra4-1
Uploaded by
ernestlambilaouarminaCopyright:
Available Formats
You might also like
- Gates Flexiva 1kwDocument142 pagesGates Flexiva 1kwJuan perezNo ratings yet
- Entalpia de Neutralizacion PDFDocument4 pagesEntalpia de Neutralizacion PDFSilvia Guerrero GonzalezNo ratings yet
- Admiralty Manual of Navigation (2003 Edition) PDFDocument131 pagesAdmiralty Manual of Navigation (2003 Edition) PDFMuak K100% (2)
- Conductivity of Strong and Weak Electrolytes With Cobra4: (Item No.: P3060660)Document9 pagesConductivity of Strong and Weak Electrolytes With Cobra4: (Item No.: P3060660)Andrew May NcubeNo ratings yet
- Column Chromatography - Separation of Leaf Pigments: Task and EquipmentDocument6 pagesColumn Chromatography - Separation of Leaf Pigments: Task and EquipmentDuc Anh NguyenNo ratings yet
- Adsorption Isotherms: (Item No.: P3040801)Document7 pagesAdsorption Isotherms: (Item No.: P3040801)Leonardo JaimesNo ratings yet
- Experiment - Determination of Faraday's ConstantDocument6 pagesExperiment - Determination of Faraday's Constantmuzahir.ali.baloch2021No ratings yet
- Viscosity Measurements With The Falling Ball Viscometer: (Item No.: P2140400)Document6 pagesViscosity Measurements With The Falling Ball Viscometer: (Item No.: P2140400)salmanNo ratings yet
- publication_10_17777_250Document5 pagespublication_10_17777_250chouhansushila08No ratings yet
- Surface Tension With The Ring Method (Du Nouy Method) : (Item No.: P2140500)Document6 pagesSurface Tension With The Ring Method (Du Nouy Method) : (Item No.: P2140500)Eduard VanegasNo ratings yet
- 18 DistillationDocument7 pages18 DistillationYvette Bitoumou Epse BileNo ratings yet
- Thermal Expansion in Solids and Liquids: (Item No.: P2310100)Document8 pagesThermal Expansion in Solids and Liquids: (Item No.: P2310100)Shera IeraNo ratings yet
- Pricelist Intralab 2015 PDFDocument50 pagesPricelist Intralab 2015 PDFNeli MunaNo ratings yet
- Supplemental Procurement Project Management Plan (PPMP) School: Catalino G. Tampipi Elementary School District: Matanao 1Document3 pagesSupplemental Procurement Project Management Plan (PPMP) School: Catalino G. Tampipi Elementary School District: Matanao 1Dahlia VillarNo ratings yet
- New BS-380 Preventive Maintenance Manual V1.0 enDocument23 pagesNew BS-380 Preventive Maintenance Manual V1.0 enBikram Thapa100% (1)
- QuoteDocument4 pagesQuoteRISHI FOOD TESTING LABNo ratings yet
- Proposed Supply, Fabrication and Installation of LPG Pipelines For Ace Medical CenterDocument7 pagesProposed Supply, Fabrication and Installation of LPG Pipelines For Ace Medical CenterWinona Marie Dohig BaquialNo ratings yet
- National Institute of Technology, Warangal - 506 004 Purchase Proposal FormDocument2 pagesNational Institute of Technology, Warangal - 506 004 Purchase Proposal FormNavin BNo ratings yet
- National Institute of Technology, Warangal - 506 004 Purchase Proposal FormDocument2 pagesNational Institute of Technology, Warangal - 506 004 Purchase Proposal FormNavin BNo ratings yet
- LEC 02.07 Determination of The Hydration Enthalpy of An ElectrolyteDocument4 pagesLEC 02.07 Determination of The Hydration Enthalpy of An ElectrolyteRoslinah SaindiNo ratings yet
- Chapter VDocument1 pageChapter VjonalyncabulloNo ratings yet
- Microbiology Section 3A Endorsement SheetDocument2 pagesMicrobiology Section 3A Endorsement SheetHades Luciferos PallonesNo ratings yet
- Proforma Invoice - 2020-0003-AEAL-1 PDFDocument4 pagesProforma Invoice - 2020-0003-AEAL-1 PDFAdnan AdamNo ratings yet
- Bromate Prove Ulr en 2016-01-06 HintDocument3 pagesBromate Prove Ulr en 2016-01-06 Hinttata_77No ratings yet
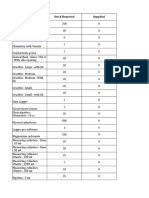
- Item - Name Stock Required SuppliedDocument6 pagesItem - Name Stock Required Suppliedraghava123456No ratings yet
- Lec04 08 LVDocument4 pagesLec04 08 LVYomaris Hernández BerríoNo ratings yet
- Solar Ray CollectorDocument4 pagesSolar Ray Collectorsaid m rauzanNo ratings yet
- Quote To JAMES JIM TIMAKADocument5 pagesQuote To JAMES JIM TIMAKAGerald ObalimNo ratings yet
- Mikeluna@momentgroup - PH: Code Description Unit Prices Amount QntyDocument2 pagesMikeluna@momentgroup - PH: Code Description Unit Prices Amount QntyHamid Alizadeh SagharlooNo ratings yet
- SMP It As-Shidqi Lab IpaDocument5 pagesSMP It As-Shidqi Lab IpaHanif AgraNo ratings yet
- Annex To The CPT Name Quantity Price On GNF: Laboratory Sieve Diameter in MM DN 250Document15 pagesAnnex To The CPT Name Quantity Price On GNF: Laboratory Sieve Diameter in MM DN 250Vijay JamadarNo ratings yet
- Bs-300 Preventive Maintenance Manual v1-1.0 enDocument19 pagesBs-300 Preventive Maintenance Manual v1-1.0 ennery castro100% (1)
- REAGENT 11 Juni 2020Document1 pageREAGENT 11 Juni 2020Andhy AlfharoNo ratings yet
- Acce (2) - 134Document1 pageAcce (2) - 134Meditech visionbdNo ratings yet
- WWW Stanhope-Seta Co UkDocument4 pagesWWW Stanhope-Seta Co Ukdennisedwin96No ratings yet
- 251-270 Test Kits MCDocument20 pages251-270 Test Kits MCluis miguel huarita castellon100% (1)
- Solutions: Glasswares & Medical Devices Price List 2018Document2 pagesSolutions: Glasswares & Medical Devices Price List 2018Ali RazaNo ratings yet
- BS-200E - Preventive Maintenance Kit Manual - V1.0 - EN - ZSH-13083-BS-200EDocument20 pagesBS-200E - Preventive Maintenance Kit Manual - V1.0 - EN - ZSH-13083-BS-200Eelias martinezNo ratings yet
- Brio 2 - 2S: Suggested Spare Parts ListDocument2 pagesBrio 2 - 2S: Suggested Spare Parts ListBrezhnev AguilarNo ratings yet
- Determining The Pka Value of A Weak Acid With Half TitrationDocument12 pagesDetermining The Pka Value of A Weak Acid With Half TitrationFranchesca BarzolaNo ratings yet
- Depo Ok NonDocument4 pagesDepo Ok NonYutmini RhisdianaNo ratings yet
- PH and Acidity of CoffeeDocument4 pagesPH and Acidity of Coffeeoshlaura0% (1)
- Endoscopy Instruments Urology090521 FINALDocument5 pagesEndoscopy Instruments Urology090521 FINALSateesh KumarNo ratings yet
- Primacs TOC Analyser: Chapter 7: Components and AccessoriesDocument6 pagesPrimacs TOC Analyser: Chapter 7: Components and AccessoriesAnonymous 2LYCWDPuiuNo ratings yet
- Materials Wet Stand PipeDocument1 pageMaterials Wet Stand PipeApril Grace BautistaNo ratings yet
- Planck's "Quantum of Action" and External Photoelectric EffectDocument8 pagesPlanck's "Quantum of Action" and External Photoelectric EffectRENZO RANIEIRO CHUMPITASI SANTANANo ratings yet
- Format Rko 2020 Dan RKBMHP 2020 PKM PaccerakkangDocument44 pagesFormat Rko 2020 Dan RKBMHP 2020 PKM PaccerakkangDiana GinaNo ratings yet
- Letter HeadDocument5 pagesLetter HeadfuentesmikeymikeNo ratings yet
- Process Safety Lab ReportDocument24 pagesProcess Safety Lab ReportTeal TealyNo ratings yet
- 2020 - Equipos de Laboratorio Ciego-2Document4 pages2020 - Equipos de Laboratorio Ciego-2Heisy Linares RodriguezNo ratings yet
- Cetak Stop Opname - AlkesDocument22 pagesCetak Stop Opname - AlkesOkto YutubNo ratings yet
- 407 SimDocument1 page407 SimYudha DorkzillaNo ratings yet
- Anggaran Lab 2018Document3 pagesAnggaran Lab 2018SpamRegional DIYNo ratings yet
- Project Proposal - Plumbing - Lab Tools and EquipmentDocument2 pagesProject Proposal - Plumbing - Lab Tools and EquipmentJOEVIN TOTESORANo ratings yet
- Proforma Invoice: Global Biotech and ConsultancyDocument2 pagesProforma Invoice: Global Biotech and ConsultancySaurabh MishraNo ratings yet
- Hach DR 900Document2 pagesHach DR 900edgar.jflores18No ratings yet
- Deep F.C KitDocument6 pagesDeep F.C KitVipul SharmaNo ratings yet
- Standar Satuan Harga Belanja Barang Tahun 2018Document5 pagesStandar Satuan Harga Belanja Barang Tahun 2018Nanang AlamsyahNo ratings yet
- List For BiochemDocument5 pagesList For Biochemajogidaniel41No ratings yet
- Instruction Manual For Zematra Mini-Lab: Order Code 1001075Document16 pagesInstruction Manual For Zematra Mini-Lab: Order Code 1001075Dka BayuNo ratings yet
- Part 5 ....Document109 pagesPart 5 ....career doctorNo ratings yet
- AtacandDocument4 pagesAtacandljubodragNo ratings yet
- KSP Solutibilty Practice ProblemsDocument22 pagesKSP Solutibilty Practice ProblemsRohan BhatiaNo ratings yet
- Eon 2019 Sustainability ReportDocument122 pagesEon 2019 Sustainability ReportDarryl Farhan WidyawanNo ratings yet
- Initial Environmental Examination (Iee) Checklist For Plastic Recycling 1.0 General InformationDocument23 pagesInitial Environmental Examination (Iee) Checklist For Plastic Recycling 1.0 General Informationavie100% (1)
- Terms ICT 1Document27 pagesTerms ICT 1Quỳnh Trang NguyễnNo ratings yet
- The Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicsDocument5 pagesThe Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicssilagadzeNo ratings yet
- 3.4 - Ferrara, Alessandro - Concept. Objections To The Notion of Phronesis. Concluding Remarks (En)Document23 pages3.4 - Ferrara, Alessandro - Concept. Objections To The Notion of Phronesis. Concluding Remarks (En)Johann Vessant Roig100% (1)
- Intravenous Fluid Therapy in Adults and ChildrenDocument16 pagesIntravenous Fluid Therapy in Adults and Childrenannisa hayati100% (1)
- Heat Tracing Iso 2Document1 pageHeat Tracing Iso 2jsmnjasmines100% (1)
- Beginner Guide - Checklist & Procedures For Ms Flight Simulator by Jaydee 1.23.2Document4 pagesBeginner Guide - Checklist & Procedures For Ms Flight Simulator by Jaydee 1.23.2XBox Aviation100% (1)
- Nondissipative Clamping Benefits DC-DC ConvertersDocument5 pagesNondissipative Clamping Benefits DC-DC ConvertersMateusz LiszczykNo ratings yet
- Tomographic Imaging in Aditya Tokamak: Nitin JainDocument21 pagesTomographic Imaging in Aditya Tokamak: Nitin JainHafiz Luqman SaifiNo ratings yet
- Sds File-16179386Document7 pagesSds File-16179386omar silimNo ratings yet
- OSHA 1926 Subpart M App C - Personal Fall Arrest Systems - Non-Mandatory Guidelines For Complying With 1926.502 (D)Document8 pagesOSHA 1926 Subpart M App C - Personal Fall Arrest Systems - Non-Mandatory Guidelines For Complying With 1926.502 (D)Gunnie PandherNo ratings yet
- Programmes For School, Strengthening Environmental Education in School SystemDocument10 pagesProgrammes For School, Strengthening Environmental Education in School SystemRuhi waliaNo ratings yet
- Engine Control Toyota Cuiser 2007 - 1GR - FeDocument6 pagesEngine Control Toyota Cuiser 2007 - 1GR - Fe35.Hoàng Xuân TânNo ratings yet
- DVS 1619 EnglishDocument20 pagesDVS 1619 EnglishSlobodan Garic50% (2)
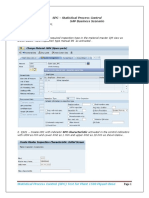
- Control Charts in SAP QM: Step by StepDocument10 pagesControl Charts in SAP QM: Step by StepPiyush BoseNo ratings yet
- Welder and Procedure QualificationDocument25 pagesWelder and Procedure QualificationRamón G. Pacheco100% (3)
- Research Article: An Improved Method of Particle Swarm Optimization For Path Planning of Mobile RobotDocument12 pagesResearch Article: An Improved Method of Particle Swarm Optimization For Path Planning of Mobile Robotmarcio pivelloNo ratings yet
- Udaipur WikiDocument19 pagesUdaipur Wikipiyush jainNo ratings yet
- © Iessg: The Global Positioning SystemDocument23 pages© Iessg: The Global Positioning SystemMiDa AlaribyNo ratings yet
- EZ9 Series HMI ManualDocument34 pagesEZ9 Series HMI ManualDanielito AlvaracinNo ratings yet
- Lichtenberg Figures - Lightning As ArtDocument14 pagesLichtenberg Figures - Lightning As ArtSyncOrSwimNo ratings yet
- Sis - SSM Price List Yarn For The Month of Feb 2024 Upto 15.02.2024Document1 pageSis - SSM Price List Yarn For The Month of Feb 2024 Upto 15.02.2024GOWRIJEYASHANKAR S KNo ratings yet
- Takeoff and Landing PerformanceDocument177 pagesTakeoff and Landing PerformanceАндрей ЛубяницкийNo ratings yet
- Buchenwald - Through The Eyes of An ArtistDocument27 pagesBuchenwald - Through The Eyes of An ArtistDennis M. Gilman100% (1)
- Foreign Part A: Fire Suppression System S/N Description Unit Foam Proportioning Unit Vertical Bladder Type Ul ListedDocument6 pagesForeign Part A: Fire Suppression System S/N Description Unit Foam Proportioning Unit Vertical Bladder Type Ul ListedNakoraNo ratings yet
P3060760 Titrage conductimétrique, avec Cobra4-1
P3060760 Titrage conductimétrique, avec Cobra4-1
Uploaded by
ernestlambilaouarminaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
P3060760 Titrage conductimétrique, avec Cobra4-1
P3060760 Titrage conductimétrique, avec Cobra4-1
Uploaded by
ernestlambilaouarminaCopyright:
Available Formats
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Conductometric titration with Cobra4 (Item No.: P3060760)
Curricular Relevance
Experiment:
Area of Expertise: Education Level: Topic: Subtopic:
Conductometric
Chemie Hochschule Analytische Chemie Titration
titration with Cobra4
Difficulty Preparation Time Execution Time Recommended Group Size
Intermediate 10 Minutes 10 Minutes 2 Students
Additional Requirements: Experiment Variations:
Precision balance, 620 g / 0.001 g
PC with USB interface, Windows XP or higher
Keywords:
electrolyte, electrical conductance, specific conductance, ion mobility, ion conductivity, conductometry, volumetry
Overview
Short description
Principle
The electric conductivity of aqueous electrolyte solutions is determined by the type and number of charge carriers at constant
temperature. Characteristic changes in conductivity are connected with changes in the ionic composition of reacting systems.
These can be used in the conductiometric titration as end point indicators.
Fig. 1: Experimental setup.
Safety instructions
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
When handling chemicals, you should wear suitable protective gloves, safety goggles, and suitable clothing. Please refer to the
appendix for detailed safety instructions.
Safety instructions
When handling chemicals, you should wear suitable protective gloves, safety goggles, and suitable clothing. Please refer to the
appendix for detailed safety instructions.
Caustic soda solution, 0.1 N
H290: May be corrosive to metals.
P234: Keep only in original container
P280: Wear protective gloves/protective clothing/eye protection/face protection.
Hydrochloric acid, 0.1 N
H290: May be corrosive to metals.
P234: Keep only in original container.
Sulphuric acid, 0.1 N
P280: Wear protective gloves/protective clothing/eye protection/face protection.
H290: May be corrosive to metals.
Barium hydroxide
H332: Harmful if inhaled.
H302: Harmful if swallowed.
H314: Causes severe skin burns and eye damage.
H332: Harmful if inhaled.
P280: Wear protective gloves/protective clothing/ eye protection/face protection.
P301+330+331: IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do –
continue rinsing.
P309+310: IF exposed or you feel unwell: Immediately call a POISON CENTER or doctor/physician.
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Equipment
Position No. Material Order No. Quantity
1 Cobra4 Wireless/USB-Link incl. USB cable 12601-10 2
2 Cobra4 Sensor-Unit Drop Counter 12636-00 1
3 Cobra4 Sensor-Unit Conductivity+ 12632-00 1
4 USB charger for Cobra4 Mobile-Link 2 and Wireless/USB-Link 07932-99 2
5 curricuLAB measureLAB 14580-61 1
6 Holder for Cobra4 with support rod 12680-00 2
7 Conductivity temperature probe Pt1000 13701-01 1
8 Magnetic stirrer without heating, 3 ltr., 230 V 35761-99 1
9 Magnetic stirring bar 30 mm, cylindrical 46299-02 1
10 Retort stand, h = 750 mm 37694-00 1
11 Right angle boss-head clamp 37697-00 3
12 Burette clamp, roller mount., 2 pl. 37720-00 1
13 Burette, lateral stopcock, Schellbach, 25 ml 36506-01 1
14 Beaker, low, BORO 3.3, 250 ml 46054-00 1
15 Weighing dishes, square shape, 84 x 84 x 24 mm, 25 pcs. 45019-25 1
16 Volumetric flask 50 ml, IGJ12/21 36547-00 1
17 Volumetric flask 100 ml, IGJ12/21 36548-00 1
18 Volumetric flask 250 ml, IGJ14/23 36550-00 1
19 Volumetric pipette, 5 ml 36577-00 3
20 Volumetric pipette, 10 ml 36578-00 2
21 Pipettor 36592-00 1
22 Pipette dish 36589-00 1
23 Funnel, glass, top dia. 55 mm 34457-00 1
24 Funnel, glass, top dia. 80 mm 34459-00 1
25 Spoon, special steel 33398-00 1
26 Wash bottle, plastic, 500 ml 33931-00 1
27 Pasteur pipettes, 250 pcs 36590-00 1
28 Rubber caps, 10 pcs 39275-03 1
29 Sulphuric acid,0.5M 1000 ml 48462-70 1
30 Hydrochloric acid,0.1M 1000 ml 48452-70 1
31 Acetic acid, 0.1 M sol., 1000 ml 48126-70 1
32 Caustic soda sol.,0.1M 1000 ml 48328-70 1
33 Barium hydroxide 250 g 30034-25 1
34 Water, distilled 5 l 31246-81 1
35 Standard solution 1413µS/cm(25°C), 460ml 47070-02 1
Tasks
Perform the following conductrometric titrations and record your measurement data with measureLAB and the
Cobra4 sensors 'Chemistry' & 'Drop counter'
a) approximately 0.1 molar barium hydroxide solution with 0.1 molar sulphuric acid,
b) approximately 0.1 molar hydrochloric acid with 0.1 molar sodium hydroxide solution and
c) approximately 0.1 molar acetic acid with 0.1 molar sodium hydroxide solution.
Other samples can alternatively be set in advance for conductometric determination of their concentration contents.
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Setup and procedure
Setup
Prepare the solutions required for the experiment as follows:
– 0.1 molar solution: Weigh 7.9 g of barium hydroxide ( ) into a 250 ml volumetric flask, add
some distilled water to dissolve it, then make up to the mark with distilled water. Should the solution be turbid (a pointer
that is present in the solution), filter it prior to use.
– 0.1 molar solution: Pipette 10 ml of 0.5 M sulphuric acid into a 50 ml volumetric flask and make up to the mark
with distilled water.
– 0.1 molar solution: Pipette 10 ml of 1.0 M acetic acid into a 100 ml volumetric flask and make up to the
mark with distilled water.
Set up the experiment as shown in Fig. 1.
Attach the burette to the retort stand using the burette holder.
Combine the Cobra4 Sensor Unit Conductivity+ and the Cobra4 Drop Counter with the Cobra4 Wireless-Links.
Attach them to the retort stand with the holders for Cobra4 and right angle clamps.
Connect the conductivity/temperature probe to the electrode input of the Cobra4 Sensor Unit Conductivity+.
Start the PC and connect it the Cobra4 Wireless Link with a USB socket or wireless connection.
After the Cobra4 Wireless-Links have been switched on, the sensors are automatically recognized. The respective ID
numbers are allocated to the sensors, which are indicated in the displays of the Cobra4 Wireless-Links.
Call up the software "measureLAB" .
Boot the experiment “Conductometric titration with Cobra4” (experiment > open experiment). The measurement
parameters for this experiment are loaded now.
Adjust the magnetic stirrer to a medium stirring speed.
For calibration: Pour some standard solution into a beaker and immerse the well-rinsed probe into the solution (Advice:
Both platinum electrodes of the probe have to be covered completely with the solution).
Go to and then click on Sensors/channels and select "Conductivity ". To perform 1-point calibration, click on
.
Enter the corrected value for the conductivity at a given temperature (cf. Fig. 2). You can find this value on the label of the
standard solution (at 25 °C with ) Click the “Apply corrected value” button and repeat this step for the
second point of calibration. Finish the calibration with “OK”.
Fig. 2: Settings for the calibration mode of the sensor.
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Procedure
Place a 250 ml glass beaker containing approximately 100 ml of distilled water and a magnetic stirrer bar on the magnetic
stirrer.
Immerse the previously well-rinsed conductivity cell in the water and fix it in one of the electrode holes of the Cobra4 Drop
Counter.
Pipette 5 ml of the 0.1 M barium hydroxide solution into the water in the beaker. Fill the burette with 0.1 M sulphuric acid
and fix it above the beaker.
Place the outlet of the burette in such a manner that the Cobra4 Drop Counter is able to record every single drop. For this
the outlet of the burette should be in the center of the light barrier.
Start the measurement with .
Add the standard solution dropwise until a total of 10 ml has been added.
Terminate the measurement with .
Save your project by clicking on the button in the top bar.
Fig. 3 shows the graph as it is presented by the program.
Carry out measurements with other acids using the same procedure.
In each case pipette 5 ml of the 0.1 M acid into the glass beaker containing 100 ml of distilled water, and fill the burette
with 0.1 M sodium hydroxide solution.
Fig. 3: Change in the specific conductivity κ in the titration of an
approximately 0.1 molar barium hydroxide solution (V = 5 ml)
with sulphuric acid (c = 0.1 mol / l).
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Theory and evaluation
The electrical conductance of an electrolytic solution and the specific conductivity calculated from it
are comprised (additively) of the different contributions of the individual ion types:
(1)
where
Cell constant (quotient of the distance and area of the electrodes in the measured cell)
Charge number
Ion mobility
Concentration of ion i
Faraday constant
According to equation (1) an increase or decrease in conductivity at constant temperature is to be expected when the
composition ( , ) of the system to be examined is changed. In the titration of a given sample of barium hydroxide solution
with sulphuric acid both ion types of the added standard solution react with the components of the solution being determined by
forming undissolved, i.e. non-dissociated products.
(2)
According to equation (1), a constant decrease in the quantity being measured to the point of neutrality (Fig. 3) results from
the total depletion of charge carriers. Subsequent to this, the ions added in excess from the sulphuric acid are no longer
converted, thus the conductivity increases approximately linearly.
In contrast, the observable decrease in the specific conductivity in the neutralisation of a strong acid ( ) with a strong base
( ) is due to the exchange of hydronium ions having high mobility ( ) compared to the
sodium ions with a low mobility ( ) according to equation (1):
(3)
In the case of overtitration, the conductivity of the concentration of the hydronium ions with high ion mobility added in excess (
) increases linearly (Fig. 4).
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Fig. 4 :Titration diagram for the neutralisation of an
approximately 0.1 molar HCl (V = 5 ml) with NaOH (c = 0.1
mol / l).
The depicted titration curve in Fig. 5 is valid for the reaction of a weak acid ( ) with a strong base ( ).
(4)
Fig. 5: Conductivity as a function of the volume of added standard
solution in the titration of an approximately 0.1 molar aceticacid
solution (V = 5 ml) with a sodium hydroxide solution (c = 0.1 mol /
l).
Due to the low autodissociation, the acid shows a comparatively low conductivity. As a result of the dissociation being
suppressed further due to salt formation, the conductivity first continues to decrease. Before reaching the point of neutrality, the
conductivity slightly increases due to the increased contribution of the sodium and acetate ions to the concentration. After the
acid has been completely transformed to the salt, the measured conductivity increases approximately linearly resulting from the
continued addition of the standard solution as discussed in the above cases due to the addition of excess titrators. The point of
neutrality can be taken from the titration curves by determining the local conductivity minimum, in the case of the acetic acid by
determining the point of intersection of the two straight lines (see Fig. 5). Use to generate regression lines with your data and
consequently, determine the cross section of the two regression lines using the tool.
The concentration and thus the quantity of ions in the given sample having a volume can be calculated from the
consumption of the known concentration of the standard solution using the relationship
(5)
where
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
Student's Sheet
Printed: 26/04/2019 13:29:00 | P3060760
Molar mass of the electrolyte to be determined
Data and results
The titration curves are shown in Figs. 3 to 5. The volumes of the standard solution required to reach the equivalence points can
be determined from them, i.e. (Fig.3), (Fig. 4) and (Fig. 5).
From equation (5), the concentrations , and
can be calculated, and from these the masses in the 5 ml test volumes;
, and .
Appendix
Disposal
The diluted and neutralised solutions of the used acids and bases can be disposed by rinsing into the drain.
Appendix
Hazard symbol, signal word Hazard statements Precautionary statements
Caustic soda solution, 0.1 N
H290: May be corrosive P234: Keep only in original container
to metals.
Attention
Hydrochloric acid, 0.1 N
H290: May be corrosive P234: Keep only in original container.
to metals.
Attention
Sulphuric acid, 0.1 N
H290: May be corrosive -
to metals.
Attention
Barium hydroxide
H332: Harmful if P280: Wear protective gloves/protective clothing/ eye protection/face
inhaled. protection.
H302: Harmful if P301+330+331: IF SWALLOWED: Rinse mouth. Do NOT induce
swallowed. vomiting.
H314: Causes severe P305+351+338: IF IN EYES: Rinse cautiously with water for several
skin burns and eye minutes. Remove contact lenses if present and easy to do – continue
Danger damage. rinsing.
P309: IF exposed or you feel unwell:
P310: Immediately call a POISON CENTER or doctor/physician.
Robert-Bosch-Breite 10 Tel: +49 551 604 - 0 info@phywe.de
D - 37079 Göttingen Fax: +49 551 604 - 107 www.phywe.com
You might also like
- Gates Flexiva 1kwDocument142 pagesGates Flexiva 1kwJuan perezNo ratings yet
- Entalpia de Neutralizacion PDFDocument4 pagesEntalpia de Neutralizacion PDFSilvia Guerrero GonzalezNo ratings yet
- Admiralty Manual of Navigation (2003 Edition) PDFDocument131 pagesAdmiralty Manual of Navigation (2003 Edition) PDFMuak K100% (2)
- Conductivity of Strong and Weak Electrolytes With Cobra4: (Item No.: P3060660)Document9 pagesConductivity of Strong and Weak Electrolytes With Cobra4: (Item No.: P3060660)Andrew May NcubeNo ratings yet
- Column Chromatography - Separation of Leaf Pigments: Task and EquipmentDocument6 pagesColumn Chromatography - Separation of Leaf Pigments: Task and EquipmentDuc Anh NguyenNo ratings yet
- Adsorption Isotherms: (Item No.: P3040801)Document7 pagesAdsorption Isotherms: (Item No.: P3040801)Leonardo JaimesNo ratings yet
- Experiment - Determination of Faraday's ConstantDocument6 pagesExperiment - Determination of Faraday's Constantmuzahir.ali.baloch2021No ratings yet
- Viscosity Measurements With The Falling Ball Viscometer: (Item No.: P2140400)Document6 pagesViscosity Measurements With The Falling Ball Viscometer: (Item No.: P2140400)salmanNo ratings yet
- publication_10_17777_250Document5 pagespublication_10_17777_250chouhansushila08No ratings yet
- Surface Tension With The Ring Method (Du Nouy Method) : (Item No.: P2140500)Document6 pagesSurface Tension With The Ring Method (Du Nouy Method) : (Item No.: P2140500)Eduard VanegasNo ratings yet
- 18 DistillationDocument7 pages18 DistillationYvette Bitoumou Epse BileNo ratings yet
- Thermal Expansion in Solids and Liquids: (Item No.: P2310100)Document8 pagesThermal Expansion in Solids and Liquids: (Item No.: P2310100)Shera IeraNo ratings yet
- Pricelist Intralab 2015 PDFDocument50 pagesPricelist Intralab 2015 PDFNeli MunaNo ratings yet
- Supplemental Procurement Project Management Plan (PPMP) School: Catalino G. Tampipi Elementary School District: Matanao 1Document3 pagesSupplemental Procurement Project Management Plan (PPMP) School: Catalino G. Tampipi Elementary School District: Matanao 1Dahlia VillarNo ratings yet
- New BS-380 Preventive Maintenance Manual V1.0 enDocument23 pagesNew BS-380 Preventive Maintenance Manual V1.0 enBikram Thapa100% (1)
- QuoteDocument4 pagesQuoteRISHI FOOD TESTING LABNo ratings yet
- Proposed Supply, Fabrication and Installation of LPG Pipelines For Ace Medical CenterDocument7 pagesProposed Supply, Fabrication and Installation of LPG Pipelines For Ace Medical CenterWinona Marie Dohig BaquialNo ratings yet
- National Institute of Technology, Warangal - 506 004 Purchase Proposal FormDocument2 pagesNational Institute of Technology, Warangal - 506 004 Purchase Proposal FormNavin BNo ratings yet
- National Institute of Technology, Warangal - 506 004 Purchase Proposal FormDocument2 pagesNational Institute of Technology, Warangal - 506 004 Purchase Proposal FormNavin BNo ratings yet
- LEC 02.07 Determination of The Hydration Enthalpy of An ElectrolyteDocument4 pagesLEC 02.07 Determination of The Hydration Enthalpy of An ElectrolyteRoslinah SaindiNo ratings yet
- Chapter VDocument1 pageChapter VjonalyncabulloNo ratings yet
- Microbiology Section 3A Endorsement SheetDocument2 pagesMicrobiology Section 3A Endorsement SheetHades Luciferos PallonesNo ratings yet
- Proforma Invoice - 2020-0003-AEAL-1 PDFDocument4 pagesProforma Invoice - 2020-0003-AEAL-1 PDFAdnan AdamNo ratings yet
- Bromate Prove Ulr en 2016-01-06 HintDocument3 pagesBromate Prove Ulr en 2016-01-06 Hinttata_77No ratings yet
- Item - Name Stock Required SuppliedDocument6 pagesItem - Name Stock Required Suppliedraghava123456No ratings yet
- Lec04 08 LVDocument4 pagesLec04 08 LVYomaris Hernández BerríoNo ratings yet
- Solar Ray CollectorDocument4 pagesSolar Ray Collectorsaid m rauzanNo ratings yet
- Quote To JAMES JIM TIMAKADocument5 pagesQuote To JAMES JIM TIMAKAGerald ObalimNo ratings yet
- Mikeluna@momentgroup - PH: Code Description Unit Prices Amount QntyDocument2 pagesMikeluna@momentgroup - PH: Code Description Unit Prices Amount QntyHamid Alizadeh SagharlooNo ratings yet
- SMP It As-Shidqi Lab IpaDocument5 pagesSMP It As-Shidqi Lab IpaHanif AgraNo ratings yet
- Annex To The CPT Name Quantity Price On GNF: Laboratory Sieve Diameter in MM DN 250Document15 pagesAnnex To The CPT Name Quantity Price On GNF: Laboratory Sieve Diameter in MM DN 250Vijay JamadarNo ratings yet
- Bs-300 Preventive Maintenance Manual v1-1.0 enDocument19 pagesBs-300 Preventive Maintenance Manual v1-1.0 ennery castro100% (1)
- REAGENT 11 Juni 2020Document1 pageREAGENT 11 Juni 2020Andhy AlfharoNo ratings yet
- Acce (2) - 134Document1 pageAcce (2) - 134Meditech visionbdNo ratings yet
- WWW Stanhope-Seta Co UkDocument4 pagesWWW Stanhope-Seta Co Ukdennisedwin96No ratings yet
- 251-270 Test Kits MCDocument20 pages251-270 Test Kits MCluis miguel huarita castellon100% (1)
- Solutions: Glasswares & Medical Devices Price List 2018Document2 pagesSolutions: Glasswares & Medical Devices Price List 2018Ali RazaNo ratings yet
- BS-200E - Preventive Maintenance Kit Manual - V1.0 - EN - ZSH-13083-BS-200EDocument20 pagesBS-200E - Preventive Maintenance Kit Manual - V1.0 - EN - ZSH-13083-BS-200Eelias martinezNo ratings yet
- Brio 2 - 2S: Suggested Spare Parts ListDocument2 pagesBrio 2 - 2S: Suggested Spare Parts ListBrezhnev AguilarNo ratings yet
- Determining The Pka Value of A Weak Acid With Half TitrationDocument12 pagesDetermining The Pka Value of A Weak Acid With Half TitrationFranchesca BarzolaNo ratings yet
- Depo Ok NonDocument4 pagesDepo Ok NonYutmini RhisdianaNo ratings yet
- PH and Acidity of CoffeeDocument4 pagesPH and Acidity of Coffeeoshlaura0% (1)
- Endoscopy Instruments Urology090521 FINALDocument5 pagesEndoscopy Instruments Urology090521 FINALSateesh KumarNo ratings yet
- Primacs TOC Analyser: Chapter 7: Components and AccessoriesDocument6 pagesPrimacs TOC Analyser: Chapter 7: Components and AccessoriesAnonymous 2LYCWDPuiuNo ratings yet
- Materials Wet Stand PipeDocument1 pageMaterials Wet Stand PipeApril Grace BautistaNo ratings yet
- Planck's "Quantum of Action" and External Photoelectric EffectDocument8 pagesPlanck's "Quantum of Action" and External Photoelectric EffectRENZO RANIEIRO CHUMPITASI SANTANANo ratings yet
- Format Rko 2020 Dan RKBMHP 2020 PKM PaccerakkangDocument44 pagesFormat Rko 2020 Dan RKBMHP 2020 PKM PaccerakkangDiana GinaNo ratings yet
- Letter HeadDocument5 pagesLetter HeadfuentesmikeymikeNo ratings yet
- Process Safety Lab ReportDocument24 pagesProcess Safety Lab ReportTeal TealyNo ratings yet
- 2020 - Equipos de Laboratorio Ciego-2Document4 pages2020 - Equipos de Laboratorio Ciego-2Heisy Linares RodriguezNo ratings yet
- Cetak Stop Opname - AlkesDocument22 pagesCetak Stop Opname - AlkesOkto YutubNo ratings yet
- 407 SimDocument1 page407 SimYudha DorkzillaNo ratings yet
- Anggaran Lab 2018Document3 pagesAnggaran Lab 2018SpamRegional DIYNo ratings yet
- Project Proposal - Plumbing - Lab Tools and EquipmentDocument2 pagesProject Proposal - Plumbing - Lab Tools and EquipmentJOEVIN TOTESORANo ratings yet
- Proforma Invoice: Global Biotech and ConsultancyDocument2 pagesProforma Invoice: Global Biotech and ConsultancySaurabh MishraNo ratings yet
- Hach DR 900Document2 pagesHach DR 900edgar.jflores18No ratings yet
- Deep F.C KitDocument6 pagesDeep F.C KitVipul SharmaNo ratings yet
- Standar Satuan Harga Belanja Barang Tahun 2018Document5 pagesStandar Satuan Harga Belanja Barang Tahun 2018Nanang AlamsyahNo ratings yet
- List For BiochemDocument5 pagesList For Biochemajogidaniel41No ratings yet
- Instruction Manual For Zematra Mini-Lab: Order Code 1001075Document16 pagesInstruction Manual For Zematra Mini-Lab: Order Code 1001075Dka BayuNo ratings yet
- Part 5 ....Document109 pagesPart 5 ....career doctorNo ratings yet
- AtacandDocument4 pagesAtacandljubodragNo ratings yet
- KSP Solutibilty Practice ProblemsDocument22 pagesKSP Solutibilty Practice ProblemsRohan BhatiaNo ratings yet
- Eon 2019 Sustainability ReportDocument122 pagesEon 2019 Sustainability ReportDarryl Farhan WidyawanNo ratings yet
- Initial Environmental Examination (Iee) Checklist For Plastic Recycling 1.0 General InformationDocument23 pagesInitial Environmental Examination (Iee) Checklist For Plastic Recycling 1.0 General Informationavie100% (1)
- Terms ICT 1Document27 pagesTerms ICT 1Quỳnh Trang NguyễnNo ratings yet
- The Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicsDocument5 pagesThe Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicssilagadzeNo ratings yet
- 3.4 - Ferrara, Alessandro - Concept. Objections To The Notion of Phronesis. Concluding Remarks (En)Document23 pages3.4 - Ferrara, Alessandro - Concept. Objections To The Notion of Phronesis. Concluding Remarks (En)Johann Vessant Roig100% (1)
- Intravenous Fluid Therapy in Adults and ChildrenDocument16 pagesIntravenous Fluid Therapy in Adults and Childrenannisa hayati100% (1)
- Heat Tracing Iso 2Document1 pageHeat Tracing Iso 2jsmnjasmines100% (1)
- Beginner Guide - Checklist & Procedures For Ms Flight Simulator by Jaydee 1.23.2Document4 pagesBeginner Guide - Checklist & Procedures For Ms Flight Simulator by Jaydee 1.23.2XBox Aviation100% (1)
- Nondissipative Clamping Benefits DC-DC ConvertersDocument5 pagesNondissipative Clamping Benefits DC-DC ConvertersMateusz LiszczykNo ratings yet
- Tomographic Imaging in Aditya Tokamak: Nitin JainDocument21 pagesTomographic Imaging in Aditya Tokamak: Nitin JainHafiz Luqman SaifiNo ratings yet
- Sds File-16179386Document7 pagesSds File-16179386omar silimNo ratings yet
- OSHA 1926 Subpart M App C - Personal Fall Arrest Systems - Non-Mandatory Guidelines For Complying With 1926.502 (D)Document8 pagesOSHA 1926 Subpart M App C - Personal Fall Arrest Systems - Non-Mandatory Guidelines For Complying With 1926.502 (D)Gunnie PandherNo ratings yet
- Programmes For School, Strengthening Environmental Education in School SystemDocument10 pagesProgrammes For School, Strengthening Environmental Education in School SystemRuhi waliaNo ratings yet
- Engine Control Toyota Cuiser 2007 - 1GR - FeDocument6 pagesEngine Control Toyota Cuiser 2007 - 1GR - Fe35.Hoàng Xuân TânNo ratings yet
- DVS 1619 EnglishDocument20 pagesDVS 1619 EnglishSlobodan Garic50% (2)
- Control Charts in SAP QM: Step by StepDocument10 pagesControl Charts in SAP QM: Step by StepPiyush BoseNo ratings yet
- Welder and Procedure QualificationDocument25 pagesWelder and Procedure QualificationRamón G. Pacheco100% (3)
- Research Article: An Improved Method of Particle Swarm Optimization For Path Planning of Mobile RobotDocument12 pagesResearch Article: An Improved Method of Particle Swarm Optimization For Path Planning of Mobile Robotmarcio pivelloNo ratings yet
- Udaipur WikiDocument19 pagesUdaipur Wikipiyush jainNo ratings yet
- © Iessg: The Global Positioning SystemDocument23 pages© Iessg: The Global Positioning SystemMiDa AlaribyNo ratings yet
- EZ9 Series HMI ManualDocument34 pagesEZ9 Series HMI ManualDanielito AlvaracinNo ratings yet
- Lichtenberg Figures - Lightning As ArtDocument14 pagesLichtenberg Figures - Lightning As ArtSyncOrSwimNo ratings yet
- Sis - SSM Price List Yarn For The Month of Feb 2024 Upto 15.02.2024Document1 pageSis - SSM Price List Yarn For The Month of Feb 2024 Upto 15.02.2024GOWRIJEYASHANKAR S KNo ratings yet
- Takeoff and Landing PerformanceDocument177 pagesTakeoff and Landing PerformanceАндрей ЛубяницкийNo ratings yet
- Buchenwald - Through The Eyes of An ArtistDocument27 pagesBuchenwald - Through The Eyes of An ArtistDennis M. Gilman100% (1)
- Foreign Part A: Fire Suppression System S/N Description Unit Foam Proportioning Unit Vertical Bladder Type Ul ListedDocument6 pagesForeign Part A: Fire Suppression System S/N Description Unit Foam Proportioning Unit Vertical Bladder Type Ul ListedNakoraNo ratings yet