Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
2 viewsMetabolism
Metabolism
Uploaded by
yakumothrylosCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Drawing Human Anatomy by Giovanni CivardiDocument89 pagesDrawing Human Anatomy by Giovanni CivardiAlex Barreto92% (88)
- Chapter 33 Management of Patients With Nonmalignant Hematologic DisordersDocument17 pagesChapter 33 Management of Patients With Nonmalignant Hematologic DisordersAira Anne Tonee Villamin100% (4)
- AP Biology Cheat Sheet PDFDocument7 pagesAP Biology Cheat Sheet PDFJ15No ratings yet
- Biochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureDocument4 pagesBiochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureJohn Daniel AriasNo ratings yet
- Biochemical Energy Production - BIOCHEMISTRYDocument3 pagesBiochemical Energy Production - BIOCHEMISTRYnezuko978No ratings yet
- Biochemistry NotesDocument2 pagesBiochemistry Notesnezuko978No ratings yet
- Biochem Final ReviewerDocument19 pagesBiochem Final RevieweryjllhallareNo ratings yet
- rev_notes_ch21_eDocument7 pagesrev_notes_ch21_ekazocheungNo ratings yet
- Biochemical Energy ProductionDocument5 pagesBiochemical Energy ProductionMaria Cyril BaldovinoNo ratings yet
- Traffic Light Topic 1 Biological MoleculesDocument3 pagesTraffic Light Topic 1 Biological MoleculesAlison HillNo ratings yet
- BioenergeticsDocument14 pagesBioenergeticsmunozayshiaNo ratings yet
- Biomolecules NcertDocument28 pagesBiomolecules NcertB. Rajeev Kungur80% (5)
- Structural Components of The Cell MembraneDocument2 pagesStructural Components of The Cell MembraneYumiNo ratings yet
- 7th Lect. 2017 MetabolismDocument46 pages7th Lect. 2017 MetabolismNedhal Mahmoud KaleefahNo ratings yet
- CHAPTER 17 The Metabolism An OverviewDocument10 pagesCHAPTER 17 The Metabolism An Overview楊畯凱No ratings yet
- Biochemistry Mini NotesDocument17 pagesBiochemistry Mini Notesdominguezangela2002No ratings yet
- Biochemical Energy ProductionDocument5 pagesBiochemical Energy ProductionAlexandria StylesNo ratings yet
- Energy TransformationDocument5 pagesEnergy TransformationmunozayshiaNo ratings yet
- Biology NotesDocument5 pagesBiology NotesFaith SNNo ratings yet
- CBSE Quick Revision Notes (Class-11 Biology) Chapter-09 BiomoleculesDocument5 pagesCBSE Quick Revision Notes (Class-11 Biology) Chapter-09 BiomoleculesSIDHARTH SBNo ratings yet
- 5 Biochemical PathwaysDocument33 pages5 Biochemical PathwaysYong AlbertoNo ratings yet
- Bio ReviewerDocument10 pagesBio ReviewerPau VillanuevaNo ratings yet
- Midterm Biochem ReviewerDocument19 pagesMidterm Biochem ReviewerNATALIE NICOLE GABASNo ratings yet
- Introduction of MetabolismDocument51 pagesIntroduction of MetabolismrahmatNo ratings yet
- Bioenergetics NotesDocument25 pagesBioenergetics Notesmissyclaudia084No ratings yet
- Nature of MetabolismDocument12 pagesNature of MetabolismIshanSaneNo ratings yet
- Biology ChecklistDocument29 pagesBiology ChecklistgsapkaiteNo ratings yet
- Metabolism (Compatibility Mode)Document13 pagesMetabolism (Compatibility Mode)Dark_KiroNo ratings yet
- Transes Chap 1 MicrobioDocument4 pagesTranses Chap 1 MicrobioFReyes KarylleNo ratings yet
- Webcontent 146 513 1 Biomolecules 20190926165314Document2 pagesWebcontent 146 513 1 Biomolecules 20190926165314Abhishek Kumar VermaNo ratings yet
- BIOCHEMISTRYDocument24 pagesBIOCHEMISTRYXyrielleNo ratings yet
- Biology Study Guide (Wheaton) Complete)Document7 pagesBiology Study Guide (Wheaton) Complete)Jireh HuangNo ratings yet
- He Cell 1Document34 pagesHe Cell 1Le Phuong LyNo ratings yet
- Microbial Metabolism and GrowthDocument6 pagesMicrobial Metabolism and GrowthKevin KevsNo ratings yet
- Macromolecules - Are Large Molecules Composed of Thousands of Covalently Connected AtomsDocument6 pagesMacromolecules - Are Large Molecules Composed of Thousands of Covalently Connected AtomsBon Joey BernestoNo ratings yet
- 3 4 Group 6 Written Report With CommentsDocument7 pages3 4 Group 6 Written Report With CommentsJanine Abiegale PeraltaNo ratings yet
- Cellular Metabolism Overview of MetabolismDocument17 pagesCellular Metabolism Overview of MetabolismNathan Louis PalacioNo ratings yet
- Bio Exam ReviewDocument17 pagesBio Exam Reviewvpcch6vj2wNo ratings yet
- Unit 1 The Basis of BiochemistryDocument34 pagesUnit 1 The Basis of Biochemistryarun231187No ratings yet
- Amino Acids are linked by peptide bonds to form formed by linking the α-carboxyl group of one amino acid to the α-amino group of another amino acid with a peptide bond (also called an amide bond)Document2 pagesAmino Acids are linked by peptide bonds to form formed by linking the α-carboxyl group of one amino acid to the α-amino group of another amino acid with a peptide bond (also called an amide bond)Noviemae TuandaNo ratings yet
- MCB201 L-TWO (General Microbiology I)Document16 pagesMCB201 L-TWO (General Microbiology I)omotolaNo ratings yet
- Biochemical Energy Production HandoutsDocument5 pagesBiochemical Energy Production Handoutssilvestre bolosNo ratings yet
- EnzymesDocument58 pagesEnzymesFateh RaufNo ratings yet
- Bioenergetika Dan MetabolismeDocument28 pagesBioenergetika Dan Metabolismehy brezzleNo ratings yet
- Biochemistry Ebook SampleDocument7 pagesBiochemistry Ebook Sampleابو عمرNo ratings yet
- Final Hope Biochemistry @CPSCBDocument200 pagesFinal Hope Biochemistry @CPSCBaryanjena12120227No ratings yet
- Lecture 5 Microbial MetabolismDocument66 pagesLecture 5 Microbial MetabolismZeynep IlkNo ratings yet
- Enzymes and Vitamins NotesDocument14 pagesEnzymes and Vitamins NotesRealyn ZambasNo ratings yet
- La Ode Muhammad Zuhdi Mulkiyan F1C116075 Faculty of Math and Science Haluoleo UniversityDocument20 pagesLa Ode Muhammad Zuhdi Mulkiyan F1C116075 Faculty of Math and Science Haluoleo UniversityMuh Zuhdi MulkianNo ratings yet
- Biochem Notes 1Document5 pagesBiochem Notes 1pumpiepumpkin12No ratings yet
- Trans Lecture Intro To BiochemDocument3 pagesTrans Lecture Intro To Biochemlovely coleenNo ratings yet
- Biochemistry CHAPTER 1Document10 pagesBiochemistry CHAPTER 1Joyce ElizaldeNo ratings yet
- Cellular MetabolismDocument79 pagesCellular MetabolismDr. Nachammai NagarajanNo ratings yet
- Midterm BiochemistryDocument12 pagesMidterm BiochemistryBiology BảoNo ratings yet
- Topic 1 LectureDocument36 pagesTopic 1 Lecturebibi.hansmuddyNo ratings yet
- EnzymesDocument18 pagesEnzymesDRMEHUL DAVE100% (1)
- Nucleotide - Monomer of Nucleic AcidsDocument2 pagesNucleotide - Monomer of Nucleic AcidsMika Sophia GonzagaNo ratings yet
- Biology Notes SLDocument69 pagesBiology Notes SLIker GNo ratings yet
- Chapter 6Document51 pagesChapter 6Naufal QaweimNo ratings yet
- ChemistryOfLife NotesDocument9 pagesChemistryOfLife NotesamvooijsNo ratings yet
- Biochem PDFDocument7 pagesBiochem PDFNhư NguyễnNo ratings yet
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- GROUP 1 - Integumentary System (Review Material)Document13 pagesGROUP 1 - Integumentary System (Review Material)SALGIE SERNALNo ratings yet
- Chapter 5 Variations in ConsciousnessDocument36 pagesChapter 5 Variations in ConsciousnessChehekNo ratings yet
- Biology Key Answers 2022Document6 pagesBiology Key Answers 2022MAHATHMA GANDINo ratings yet
- Grade 11 - Life Science - Revision Questions - AnimalDocument18 pagesGrade 11 - Life Science - Revision Questions - AnimalJustinCase1991No ratings yet
- Herbs For The Urinary Tract (Michael Moore)Document187 pagesHerbs For The Urinary Tract (Michael Moore)ANGELO NHAR100% (1)
- Health Optimizing Physical Education 11 (H.O.P.E. 1) : Energy SystemDocument14 pagesHealth Optimizing Physical Education 11 (H.O.P.E. 1) : Energy SystemSean Andreson Mabalacad100% (2)
- Cambridge Assessment International Education: Biology 9700/41 May/June 2018Document17 pagesCambridge Assessment International Education: Biology 9700/41 May/June 2018shabanaNo ratings yet
- Panduan Pengajaran Dan Pembelajaran PDP KSSR Semakan 2017 Sains Tahun 4 Versi Bahasa Inggeris-CompressedDocument112 pagesPanduan Pengajaran Dan Pembelajaran PDP KSSR Semakan 2017 Sains Tahun 4 Versi Bahasa Inggeris-CompressedNur Adila Binti AzmanNo ratings yet
- Review 1 Air Con and RefDocument12 pagesReview 1 Air Con and Refrobsonrober83No ratings yet
- LPS Stimulation of THPDocument18 pagesLPS Stimulation of THPMuniba SiddiquiNo ratings yet
- BIOL 373 Midterm 2Document15 pagesBIOL 373 Midterm 2akyelamarthyNo ratings yet
- SFB1001 Biology Principles I Version 2020 Simplified 7 WeeksDocument11 pagesSFB1001 Biology Principles I Version 2020 Simplified 7 WeeksAleeya JulitaNo ratings yet
- SGS GM Lecture 8-12Document9 pagesSGS GM Lecture 8-12SleepyHead ˋωˊNo ratings yet
- Central Nervous System CROSSWORDDocument2 pagesCentral Nervous System CROSSWORDJULIO CANIPASNo ratings yet
- Pelvis 1Document10 pagesPelvis 1Nur Liyana MohamadNo ratings yet
- Cell, Microscopy, EpitheliumDocument14 pagesCell, Microscopy, EpitheliumNICOLE JADE PINEDANo ratings yet
- Activity 3B - Animal Tissues (Muscular & Nervous Tissue) - Activity SheetDocument8 pagesActivity 3B - Animal Tissues (Muscular & Nervous Tissue) - Activity SheetKristine VibarNo ratings yet
- Grade 9 Chapter 1 Revision Worksheet - 1Document3 pagesGrade 9 Chapter 1 Revision Worksheet - 1Abdullah AkmalNo ratings yet
- Chapter 3 Diffusion & Osmosis - WorksheetDocument3 pagesChapter 3 Diffusion & Osmosis - Worksheetapi-3728508100% (7)
- Overview Sistem SarafDocument55 pagesOverview Sistem Sarafluminto 102016073No ratings yet
- Skin ProjectDocument16 pagesSkin ProjectMonique WilliamsNo ratings yet
- Dentinogingival-Junction - 1Document26 pagesDentinogingival-Junction - 1drrnonhelmyNo ratings yet
- 15th Week Minerals 2023Document40 pages15th Week Minerals 2023VizhiNo ratings yet
- SyllabusDocument26 pagesSyllabuselizabethkaniparampilNo ratings yet
- Anh Van - LANGUAGE PRACTICE 2Document9 pagesAnh Van - LANGUAGE PRACTICE 2thi toNo ratings yet
- Lesson - Cell Division - DifferentiationDocument8 pagesLesson - Cell Division - DifferentiationramyaNo ratings yet
- Gram-Negative Rods Related To The Enteric Tract The EnterobacteriaceaeDocument47 pagesGram-Negative Rods Related To The Enteric Tract The EnterobacteriaceaeShattered SoulNo ratings yet
- 2021 Expt 11 Pre Lab - ChromatographyDocument4 pages2021 Expt 11 Pre Lab - ChromatographyNUR NAJWA BINTI MOHD RAFIE MoeNo ratings yet
Metabolism
Metabolism
Uploaded by
yakumothrylos0 ratings0% found this document useful (0 votes)
2 views4 pagesCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
2 views4 pagesMetabolism
Metabolism
Uploaded by
yakumothrylosCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 4
Metabolism
Metabolism is the sum total of all the biochemical
reactions that take place in a living organism.
Even the simplest living cell is continually carrying on
energy-demanding processes such as protein
synthesis, DNA replication, RNA transcription, and
membrane transport.
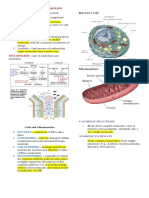
Metabolism and Cell Structure
Prokaryotic cells have no nucleus and are found only
in bacteria.
A eukaryotic cell is a cell in which the DNA is found in
a membrane-enclosed nucleus.
- Cells of this type, which are found in all higher
organisms, are about 1000 times larger than
bacterial cells.
Metabolic reactions fall into one of two subtypes:
Eukaryotic Cell Organelles and Their Function
Catabolism is all metabolic reactions in which large
biochemical molecules are broken down to smaller
ones. (CL)
- Catabolic reactions release energy.
Anabolism is all metabolic reactions in which small
biochemical molecules are joined together to form
larger ones. (AS)
- Anabolic reactions require energy in order to
proceed.
The metabolic reactions that occur in a cell are
Nucleus: DNA replication and RNA synthesis
usually organized into sequences called metabolic
pathways. Plasma membrane: Cellular boundary
Metabolic Pathway: Series of consecutive Cytoplasm: The water-based material of a eukaryotic
biochemical reactions used to convert a starting cell
material into an end product.
Mitochondria: Generates most of the energy needed
There are two types of metabolic pathways for cell.
- Linear Lysosome: Contain hydrolytic enzymes needed for
- Cyclic cell rebuilding, repair and degradation
The major pathways for all forms of life are similar: Ribosome: Sites for protein synthesis
Mitochondria . An organelle that is responsible for the
generation of most of the energy for a cell: -
Outer membrane: Permeable to small molecules: A typical cellular reaction in which ATP functions as
50% lipid, 50% protein both a source of a phosphate group and a source of
energy is the conversion of glucose to glucose 6-
Inner membrane: Highly impermeable to most
phosphate.
substances: 20% lipid, 80% protein
Inner membrane folded into cristae to increase
surface area
Synthesis of ATP occurs on the inner membrane.
Flavin Adenine Dinucleotide (FAD, FADH2)
Ribitol is a reduced form of ribose; a CH2OH group is
present in place of the -CHO group.
Flavin adenine
dinucleotide has two forms—an oxidized form and a
reduced form.
Important Nucleotide-Containing Compounds in
Metabolic Pathways
Adenosine Phosphates (ATP, ADP, and AMP)
Nicotinamide Adenine Dinucleotide (NAD+, NADH)
Emphasizes the dinucleotide nature of the coenzyme,
as well as the origin of its name, is
The phosphate–ribose bond is a phosphoester bond,
The phosphate–phosphate bonds are
phosphoanhydride bonds.
A phosphoanhydride bond is the chemical bond
NAD+
formed when two phosphate groups react with each
other and a water molecule is produced. - The + sign refers to the positive charge on the
nitrogen atom in the nicotinamide
component of the structure; this nitrogen
atom has four bonds instead of the usual
three.
NAD+ is reduced to NADH. The equation for this
change is
Note that there is no positively charged nitrogen
atom in NADH because of the second electron added.
Coenzyme A (CoA–SH)
coenzyme A (CoA), a derivative of the B vitamin
pantothenic acid.
The active portion of coenzyme A is the sulfhydryl
group (-SH group).
Classification of Metabolic Intermediate Compounds
The metabolic intermediate compounds considered in
this section can be classified into three groups based
on function.
High-Energy Phosphate Compounds
A high-energy compound is a compound that has a
greater free energy of hydrolysis than that of a
typical compound.
High-energy compounds differ from other compounds
in that they contain one or more very reactive bonds,
often called strained bonds.
The more negative the free energy of hydrolysis, the
greater the bond strain.
Important Carboxylate Ions in Metabolic Pathways
An Overview of Biochemical Energy Production transport chain, and the oxidative
phosphorylation
Stage 1. Digestion
- Begins in mouth (saliva contains starch
digesting enzymes), continues in the stomach
(gastric juice), completed in small intestine:
Results in small molecules that can cross
intestinal membrane into the blood.
End Products of digestion:
- Glucose and monosaccharides from
carbohydrates
- Amino acids from proteins
- Fatty acids and glycerol from fats and oils 1041
The digestion products are absorbed into the blood
and transported to body's cells.
Stage 2. Acetyl Group Formation
- The small molecules from Stage 1 are further
oxidized.
- End product of these oxidations is acetyl CoA
- Primary products include two-carbon acetyl
units (which become attached to coenzyme A
to give acetyl CoA) and the reduced coenzyme
NADH.
- Involves numerous reactions:
Reactions occur both in cytosol (glucose
metabolism) as well as mitochondria (fatty
acid metabolism) of the cells.
Stage 3. Citric Acid Cycle
- Takes place inside the mitochondria
- In this stage acetyl group is oxidized to
produce CO2 (which we exhale during
breathing) and energy
- Most energy is trapped and carried by the
reduced coenzymes NADH and FADH₂ to the
fourth stage
- Some energy produced in this stage is lost in
the form of heat
Stage 4. Electron Transport Chain and Oxidative
Phosphorylation .
- Takes place in mitochondria
- NADH and FADH2 are oxidized to release
- H+ and electrons H+ are transported to the
inter-membrane space in mitochondria
Electrons are transferred to O₂ and O₂ is
reduced to H₂O 2 H+ ions reenter the
mitochondrial matrix and drive
- ATP-synthase reaction to produce ATP ATP is
the primary energy carrier in metabolic
pathways
- The reactions in stages 3 & 4 are common to
the processing of carbohydrates, fats, and
proteins
- Collectively known as the common metabolic
pathways, i.e., the sum of the reactions that
occur in the citric acid cycle, the electron
You might also like
- Drawing Human Anatomy by Giovanni CivardiDocument89 pagesDrawing Human Anatomy by Giovanni CivardiAlex Barreto92% (88)
- Chapter 33 Management of Patients With Nonmalignant Hematologic DisordersDocument17 pagesChapter 33 Management of Patients With Nonmalignant Hematologic DisordersAira Anne Tonee Villamin100% (4)
- AP Biology Cheat Sheet PDFDocument7 pagesAP Biology Cheat Sheet PDFJ15No ratings yet
- Biochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureDocument4 pagesBiochemistry - Lecture Term01: Introduction To Metabolism Metabolism Metabolism and Cell StructureJohn Daniel AriasNo ratings yet
- Biochemical Energy Production - BIOCHEMISTRYDocument3 pagesBiochemical Energy Production - BIOCHEMISTRYnezuko978No ratings yet
- Biochemistry NotesDocument2 pagesBiochemistry Notesnezuko978No ratings yet
- Biochem Final ReviewerDocument19 pagesBiochem Final RevieweryjllhallareNo ratings yet
- rev_notes_ch21_eDocument7 pagesrev_notes_ch21_ekazocheungNo ratings yet
- Biochemical Energy ProductionDocument5 pagesBiochemical Energy ProductionMaria Cyril BaldovinoNo ratings yet
- Traffic Light Topic 1 Biological MoleculesDocument3 pagesTraffic Light Topic 1 Biological MoleculesAlison HillNo ratings yet
- BioenergeticsDocument14 pagesBioenergeticsmunozayshiaNo ratings yet
- Biomolecules NcertDocument28 pagesBiomolecules NcertB. Rajeev Kungur80% (5)
- Structural Components of The Cell MembraneDocument2 pagesStructural Components of The Cell MembraneYumiNo ratings yet
- 7th Lect. 2017 MetabolismDocument46 pages7th Lect. 2017 MetabolismNedhal Mahmoud KaleefahNo ratings yet
- CHAPTER 17 The Metabolism An OverviewDocument10 pagesCHAPTER 17 The Metabolism An Overview楊畯凱No ratings yet
- Biochemistry Mini NotesDocument17 pagesBiochemistry Mini Notesdominguezangela2002No ratings yet
- Biochemical Energy ProductionDocument5 pagesBiochemical Energy ProductionAlexandria StylesNo ratings yet
- Energy TransformationDocument5 pagesEnergy TransformationmunozayshiaNo ratings yet
- Biology NotesDocument5 pagesBiology NotesFaith SNNo ratings yet
- CBSE Quick Revision Notes (Class-11 Biology) Chapter-09 BiomoleculesDocument5 pagesCBSE Quick Revision Notes (Class-11 Biology) Chapter-09 BiomoleculesSIDHARTH SBNo ratings yet
- 5 Biochemical PathwaysDocument33 pages5 Biochemical PathwaysYong AlbertoNo ratings yet
- Bio ReviewerDocument10 pagesBio ReviewerPau VillanuevaNo ratings yet
- Midterm Biochem ReviewerDocument19 pagesMidterm Biochem ReviewerNATALIE NICOLE GABASNo ratings yet
- Introduction of MetabolismDocument51 pagesIntroduction of MetabolismrahmatNo ratings yet
- Bioenergetics NotesDocument25 pagesBioenergetics Notesmissyclaudia084No ratings yet
- Nature of MetabolismDocument12 pagesNature of MetabolismIshanSaneNo ratings yet
- Biology ChecklistDocument29 pagesBiology ChecklistgsapkaiteNo ratings yet
- Metabolism (Compatibility Mode)Document13 pagesMetabolism (Compatibility Mode)Dark_KiroNo ratings yet
- Transes Chap 1 MicrobioDocument4 pagesTranses Chap 1 MicrobioFReyes KarylleNo ratings yet
- Webcontent 146 513 1 Biomolecules 20190926165314Document2 pagesWebcontent 146 513 1 Biomolecules 20190926165314Abhishek Kumar VermaNo ratings yet
- BIOCHEMISTRYDocument24 pagesBIOCHEMISTRYXyrielleNo ratings yet
- Biology Study Guide (Wheaton) Complete)Document7 pagesBiology Study Guide (Wheaton) Complete)Jireh HuangNo ratings yet
- He Cell 1Document34 pagesHe Cell 1Le Phuong LyNo ratings yet
- Microbial Metabolism and GrowthDocument6 pagesMicrobial Metabolism and GrowthKevin KevsNo ratings yet
- Macromolecules - Are Large Molecules Composed of Thousands of Covalently Connected AtomsDocument6 pagesMacromolecules - Are Large Molecules Composed of Thousands of Covalently Connected AtomsBon Joey BernestoNo ratings yet
- 3 4 Group 6 Written Report With CommentsDocument7 pages3 4 Group 6 Written Report With CommentsJanine Abiegale PeraltaNo ratings yet
- Cellular Metabolism Overview of MetabolismDocument17 pagesCellular Metabolism Overview of MetabolismNathan Louis PalacioNo ratings yet
- Bio Exam ReviewDocument17 pagesBio Exam Reviewvpcch6vj2wNo ratings yet
- Unit 1 The Basis of BiochemistryDocument34 pagesUnit 1 The Basis of Biochemistryarun231187No ratings yet
- Amino Acids are linked by peptide bonds to form formed by linking the α-carboxyl group of one amino acid to the α-amino group of another amino acid with a peptide bond (also called an amide bond)Document2 pagesAmino Acids are linked by peptide bonds to form formed by linking the α-carboxyl group of one amino acid to the α-amino group of another amino acid with a peptide bond (also called an amide bond)Noviemae TuandaNo ratings yet
- MCB201 L-TWO (General Microbiology I)Document16 pagesMCB201 L-TWO (General Microbiology I)omotolaNo ratings yet
- Biochemical Energy Production HandoutsDocument5 pagesBiochemical Energy Production Handoutssilvestre bolosNo ratings yet
- EnzymesDocument58 pagesEnzymesFateh RaufNo ratings yet
- Bioenergetika Dan MetabolismeDocument28 pagesBioenergetika Dan Metabolismehy brezzleNo ratings yet
- Biochemistry Ebook SampleDocument7 pagesBiochemistry Ebook Sampleابو عمرNo ratings yet
- Final Hope Biochemistry @CPSCBDocument200 pagesFinal Hope Biochemistry @CPSCBaryanjena12120227No ratings yet
- Lecture 5 Microbial MetabolismDocument66 pagesLecture 5 Microbial MetabolismZeynep IlkNo ratings yet
- Enzymes and Vitamins NotesDocument14 pagesEnzymes and Vitamins NotesRealyn ZambasNo ratings yet
- La Ode Muhammad Zuhdi Mulkiyan F1C116075 Faculty of Math and Science Haluoleo UniversityDocument20 pagesLa Ode Muhammad Zuhdi Mulkiyan F1C116075 Faculty of Math and Science Haluoleo UniversityMuh Zuhdi MulkianNo ratings yet
- Biochem Notes 1Document5 pagesBiochem Notes 1pumpiepumpkin12No ratings yet
- Trans Lecture Intro To BiochemDocument3 pagesTrans Lecture Intro To Biochemlovely coleenNo ratings yet
- Biochemistry CHAPTER 1Document10 pagesBiochemistry CHAPTER 1Joyce ElizaldeNo ratings yet
- Cellular MetabolismDocument79 pagesCellular MetabolismDr. Nachammai NagarajanNo ratings yet
- Midterm BiochemistryDocument12 pagesMidterm BiochemistryBiology BảoNo ratings yet
- Topic 1 LectureDocument36 pagesTopic 1 Lecturebibi.hansmuddyNo ratings yet
- EnzymesDocument18 pagesEnzymesDRMEHUL DAVE100% (1)
- Nucleotide - Monomer of Nucleic AcidsDocument2 pagesNucleotide - Monomer of Nucleic AcidsMika Sophia GonzagaNo ratings yet
- Biology Notes SLDocument69 pagesBiology Notes SLIker GNo ratings yet
- Chapter 6Document51 pagesChapter 6Naufal QaweimNo ratings yet
- ChemistryOfLife NotesDocument9 pagesChemistryOfLife NotesamvooijsNo ratings yet
- Biochem PDFDocument7 pagesBiochem PDFNhư NguyễnNo ratings yet
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- GROUP 1 - Integumentary System (Review Material)Document13 pagesGROUP 1 - Integumentary System (Review Material)SALGIE SERNALNo ratings yet
- Chapter 5 Variations in ConsciousnessDocument36 pagesChapter 5 Variations in ConsciousnessChehekNo ratings yet
- Biology Key Answers 2022Document6 pagesBiology Key Answers 2022MAHATHMA GANDINo ratings yet
- Grade 11 - Life Science - Revision Questions - AnimalDocument18 pagesGrade 11 - Life Science - Revision Questions - AnimalJustinCase1991No ratings yet
- Herbs For The Urinary Tract (Michael Moore)Document187 pagesHerbs For The Urinary Tract (Michael Moore)ANGELO NHAR100% (1)
- Health Optimizing Physical Education 11 (H.O.P.E. 1) : Energy SystemDocument14 pagesHealth Optimizing Physical Education 11 (H.O.P.E. 1) : Energy SystemSean Andreson Mabalacad100% (2)
- Cambridge Assessment International Education: Biology 9700/41 May/June 2018Document17 pagesCambridge Assessment International Education: Biology 9700/41 May/June 2018shabanaNo ratings yet
- Panduan Pengajaran Dan Pembelajaran PDP KSSR Semakan 2017 Sains Tahun 4 Versi Bahasa Inggeris-CompressedDocument112 pagesPanduan Pengajaran Dan Pembelajaran PDP KSSR Semakan 2017 Sains Tahun 4 Versi Bahasa Inggeris-CompressedNur Adila Binti AzmanNo ratings yet
- Review 1 Air Con and RefDocument12 pagesReview 1 Air Con and Refrobsonrober83No ratings yet
- LPS Stimulation of THPDocument18 pagesLPS Stimulation of THPMuniba SiddiquiNo ratings yet
- BIOL 373 Midterm 2Document15 pagesBIOL 373 Midterm 2akyelamarthyNo ratings yet
- SFB1001 Biology Principles I Version 2020 Simplified 7 WeeksDocument11 pagesSFB1001 Biology Principles I Version 2020 Simplified 7 WeeksAleeya JulitaNo ratings yet
- SGS GM Lecture 8-12Document9 pagesSGS GM Lecture 8-12SleepyHead ˋωˊNo ratings yet
- Central Nervous System CROSSWORDDocument2 pagesCentral Nervous System CROSSWORDJULIO CANIPASNo ratings yet
- Pelvis 1Document10 pagesPelvis 1Nur Liyana MohamadNo ratings yet
- Cell, Microscopy, EpitheliumDocument14 pagesCell, Microscopy, EpitheliumNICOLE JADE PINEDANo ratings yet
- Activity 3B - Animal Tissues (Muscular & Nervous Tissue) - Activity SheetDocument8 pagesActivity 3B - Animal Tissues (Muscular & Nervous Tissue) - Activity SheetKristine VibarNo ratings yet
- Grade 9 Chapter 1 Revision Worksheet - 1Document3 pagesGrade 9 Chapter 1 Revision Worksheet - 1Abdullah AkmalNo ratings yet
- Chapter 3 Diffusion & Osmosis - WorksheetDocument3 pagesChapter 3 Diffusion & Osmosis - Worksheetapi-3728508100% (7)
- Overview Sistem SarafDocument55 pagesOverview Sistem Sarafluminto 102016073No ratings yet
- Skin ProjectDocument16 pagesSkin ProjectMonique WilliamsNo ratings yet
- Dentinogingival-Junction - 1Document26 pagesDentinogingival-Junction - 1drrnonhelmyNo ratings yet
- 15th Week Minerals 2023Document40 pages15th Week Minerals 2023VizhiNo ratings yet
- SyllabusDocument26 pagesSyllabuselizabethkaniparampilNo ratings yet
- Anh Van - LANGUAGE PRACTICE 2Document9 pagesAnh Van - LANGUAGE PRACTICE 2thi toNo ratings yet
- Lesson - Cell Division - DifferentiationDocument8 pagesLesson - Cell Division - DifferentiationramyaNo ratings yet
- Gram-Negative Rods Related To The Enteric Tract The EnterobacteriaceaeDocument47 pagesGram-Negative Rods Related To The Enteric Tract The EnterobacteriaceaeShattered SoulNo ratings yet
- 2021 Expt 11 Pre Lab - ChromatographyDocument4 pages2021 Expt 11 Pre Lab - ChromatographyNUR NAJWA BINTI MOHD RAFIE MoeNo ratings yet