Professional Documents
Culture Documents
65dd35da0d3bb300181ed15a_##_Chemical Kinetics Short Notes
65dd35da0d3bb300181ed15a_##_Chemical Kinetics Short Notes
Uploaded by
venombobble0 ratings0% found this document useful (0 votes)
2 views2 pagesChemical kinetics short notes
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentChemical kinetics short notes
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
2 views2 pages65dd35da0d3bb300181ed15a_##_Chemical Kinetics Short Notes
65dd35da0d3bb300181ed15a_##_Chemical Kinetics Short Notes
Uploaded by
venombobbleChemical kinetics short notes
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
CHAPTER
4 Chemical Kinetics
Chemical Kinetics In Collision Theory Activation Energy
Chemical Kinetics is the study of chemical reactions with respect In collision theory activation energy and proper orientation of the
to reaction rates, effect of various variables rearrangement of molecules together determine the criteria for an effective collision
atoms and formation of intermediates. and hence rate of a reaction.
The rate of a reaction
Nuclear Chemistry
The rate of a reaction is concerned with decrease in concentration
Nuclear chemistry is the study of chemical reactions involving
of reactants or increase in concentration of products per unit time.
changes in nuclei of atoms. It provides information about kinetic
It can be expressed as an instantaneous rate at a particular instant
of radioactive decay and time period (unaffected by temperature,
of time and average rate over a large period of time.
pressure catalyst).
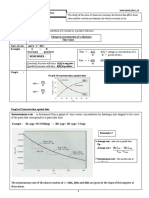
Factors affecting rate of reaction
A number of factors such as nature of reactants and products, Formulae Chart
exposure to radiations, temperature, concentration of reactants, (i) For a general chemical transformation
catalyst and surface area of solid reactants effect the rate of a nA + mB → pC + qD
reaction.
1 d[A] 1 d[B] 1 d[C] 1 d[D]
Rate =
Rate Law n dt m dt p dt q dt
Rate law is the mathematical representation for rate of reaction, it (ii) For general chemical changes
can be determined experimentally and cannot be predicted.
mA + nB → Products
Order of a reaction
dx
Order of a reaction with respect to a reactant is the power of Theoretical rate
= k[A]m [B]n
dt
its concentration term which appears in the rate law, it is an
(iii) For a general reaction:
experimentally determined quantity.
aA + bB → Products
Molecularity dx
Rate
= k[A]m [B]n
Molecularity of a reaction is the number of reacting species taking dt
part in an elementary reaction, which must collide simultaneously Order of reaction w.r.t. A = m
in order to bring about a chemical reaction. Molecularity of Order of reaction w.r.t. B = n
a reactant greater than 3 are rare, order and molecularity of
(iv) Unit of rate constant = (mole litre–1)1–n (time–1)
elementary reactions are same.
where, n = order of reaction
Rate Constant (v) For a zero order reaction: A → B
Rate constant is the proprotionality constant in rate law. d[A] 0
Rate = k[A] k(constant)
dt
Integrated Rate
(vi) For a first order reaction A → B
Integrated rate equations can be determined by integrating the
d[A]
differential rate equations. Rate = k[A]
dt
Half Life 2.303 [A]0 2.303 a
= k = log10 log10
Half life is the time, in which half of the initial amount of reactants t [A]t t a − x
is converted into products. (Time taken for 50% completion of
reactions) (vii) For a first order reaction, A → B
−k k T +10
log=
10 [A]t t + log10 [A]0 (xii) Temperature coefficient = = 2 to 3
2.303 kT
[A]0 Arrhenius equation, k = Ae − Ea / RT
(viii) For a zero order reaction t1/ 2 =
2k
0.693 k Ea T2 − T1
log10 2 =
For a first order reaction t1/ 2 =
k k [2.303R] T1T2
1
1 (A = Arrhenius’s constant Ea = Activation energy)
For an nth order reaction, t1/ 2 = (for n ≥ 2)
[A]0n −1
(ix) For a parallel reaction Ea
log
= 10 k log10 A −
2.303RT
(xiii) Binding energy, B.E. = ∆m×931.5 MeV
−d[A]
∆m = mass defect = calculated At. mass – observed At mass
= (k1 + k 2 )[A]
dt B.E.(total)
(x) For a first order reaction: A→B+C a reactant reacts with as B.E. per nucleon =
mass number
A, B and C
1 Mev = 9.6 × 1010 joule mol–1
2.303 V − V0
k=
log10 ∞
t V∞ − Vt (xiv) Packing fraction, P.F.
(V = vol. of reagent) isotopic atomic mass − mass number
= × 104
mass number
(xi) For hydrolysis of sucrose, S
+
H
→G + F (xv) In a radioactive decay, Nt = N0e–λt
2.303 r − r
k= log10 0 ∞ . Amount of radioactive substance after ‘n’ half life periods
t rt − r∞
n
1
(r = rotation due to all S, G and F) N = N0
2
P
W Chemical Kinetics 9
You might also like
- KFT 233 Chemical Kinetics - Student PDFDocument99 pagesKFT 233 Chemical Kinetics - Student PDFRody MahadiNo ratings yet
- Background Study On Continuously Stirred Tank ReactorDocument4 pagesBackground Study On Continuously Stirred Tank ReactorSyazani HussainiNo ratings yet
- Liver Enzyme LabDocument2 pagesLiver Enzyme LabskeltenboiNo ratings yet
- Chemical Kinetics _ Short NotesDocument2 pagesChemical Kinetics _ Short Notesanudiv2427No ratings yet
- Chemical Kinetics - Short NotesDocument2 pagesChemical Kinetics - Short Notesrjrahul453lNo ratings yet
- Chemical KineticsDocument10 pagesChemical KineticsMarvin JeaNo ratings yet
- Chemical Kinetics Neet MCQDocument12 pagesChemical Kinetics Neet MCQmanan10jas1529No ratings yet
- Chemical KineticsDocument33 pagesChemical KineticsHarshita&C SaysNo ratings yet
- Chemical KineticszzDocument29 pagesChemical KineticszzfailurewasteworthlessNo ratings yet
- Intensive Revision Program of Physical Chemistry: By: Brijesh Jindal SirDocument12 pagesIntensive Revision Program of Physical Chemistry: By: Brijesh Jindal SirHudsun HornetNo ratings yet
- Chemical Kinetics Bitsat 2024Document8 pagesChemical Kinetics Bitsat 2024Vedika ShahNo ratings yet
- SY - PP II - Drug StabilityDocument49 pagesSY - PP II - Drug StabilityKevalNo ratings yet
- Chemical Kinetics 1Document4 pagesChemical Kinetics 1deepanjan881No ratings yet
- Chemical KineticsDocument32 pagesChemical KineticsRaju SinghNo ratings yet
- Chemical KineticsDocument64 pagesChemical KineticsFerdiansyah SetiawanNo ratings yet
- Chapter Chemical Kinetics CHE 121Document38 pagesChapter Chemical Kinetics CHE 121iqbal-cheNo ratings yet
- Chem Chap 4 PDFDocument62 pagesChem Chap 4 PDFNur Husnina HussinNo ratings yet
- Chemical Kinetics - CBSE Sol File Ex-1Document5 pagesChemical Kinetics - CBSE Sol File Ex-1PriyanshiNo ratings yet
- Chemical Kinetics VcpucDocument12 pagesChemical Kinetics VcpucsanjanaralleNo ratings yet
- Chemical KineticsDocument32 pagesChemical KineticsTimothy HandokoNo ratings yet
- Chemical KineticsDocument21 pagesChemical Kineticsdipankargh48No ratings yet
- Chemical Kinetics: T 0 ' o D (R) D (P) R DT DTDocument12 pagesChemical Kinetics: T 0 ' o D (R) D (P) R DT DTVeerNo ratings yet
- Chemical Kinetics: Rate of A ReactionDocument49 pagesChemical Kinetics: Rate of A ReactionVijay KumarNo ratings yet
- Chemical KineticsDocument15 pagesChemical KineticsThara BijuNo ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsMikey Bryant BonbonNo ratings yet
- Chemical Reactions Rate & Activation EnergyDocument14 pagesChemical Reactions Rate & Activation EnergyNikita SinghNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Chemical Kinetics PDFDocument23 pagesCBSE Class 12 Chem Notes Question Bank Chemical Kinetics PDFAshika D ChandavarkarNo ratings yet
- Chapter 10Document158 pagesChapter 10Hafizszul FeyzulNo ratings yet
- Chemical KineticsDocument7 pagesChemical KineticshamsiniyvreddyNo ratings yet
- Kinetika ReaksiDocument46 pagesKinetika ReaksiZaenal Arifin Misgi Candra DasaNo ratings yet
- Chemical KineticDocument44 pagesChemical KineticVibhor100% (1)
- Instantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveDocument13 pagesInstantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveSupia NazmaNo ratings yet
- Chemical KineticsDocument49 pagesChemical KineticsS KNo ratings yet
- Gen Chem ReviewerDocument9 pagesGen Chem ReviewerMalayao, Philip Jude M.No ratings yet
- Chemical Kinetics and ColloidsDocument6 pagesChemical Kinetics and Colloidstahasheikh822No ratings yet
- 1.0 Reaction Kinetic 22 - 23 (REVIEWED)Document115 pages1.0 Reaction Kinetic 22 - 23 (REVIEWED)alyaainsyirah04No ratings yet
- Essentials of Chemical KineticsDocument49 pagesEssentials of Chemical KineticsJohn KanteNo ratings yet
- Chemical Kinetics 13thDocument32 pagesChemical Kinetics 13thRaju SinghNo ratings yet
- Revision Questions Chapter 4 Chemical KineticsDocument23 pagesRevision Questions Chapter 4 Chemical Kineticssimple student akashNo ratings yet
- Chemical Kinetics Lecture 1 2Document59 pagesChemical Kinetics Lecture 1 2BaNcHoNo ratings yet
- Chemical Kinetics - Reaction RatesDocument35 pagesChemical Kinetics - Reaction RatesRichie SuyaoNo ratings yet
- C1 Reaction KineticsDocument12 pagesC1 Reaction KineticsChloeNo ratings yet
- Reaction Kinetics-1Document29 pagesReaction Kinetics-1Henry GreysonNo ratings yet
- Chemical Kinetic Isotope EffectDocument43 pagesChemical Kinetic Isotope Effecttumman lal SahuNo ratings yet
- Chemical Kinetic - Dec2016 PDFDocument137 pagesChemical Kinetic - Dec2016 PDFFaisal AzamNo ratings yet
- Chemical KineticsDocument43 pagesChemical KineticsJohn SlyNo ratings yet
- Aqa 1 5Document19 pagesAqa 1 5leonidas.wujieweiNo ratings yet
- Enzyme KineticsDocument28 pagesEnzyme Kineticsghislain22.kevinNo ratings yet
- Chemical Kinetics: Gist of The LessonDocument34 pagesChemical Kinetics: Gist of The Lessonanshikahp1No ratings yet
- Reactor Technology 6Document13 pagesReactor Technology 6Sami WhiteNo ratings yet
- Chemical Kinetics - Reaction Orders: H° For This Reaction Is: H H HDocument13 pagesChemical Kinetics - Reaction Orders: H° For This Reaction Is: H H HLinaGeneyReyesNo ratings yet
- 5 1 1 Revision Guide How FastDocument7 pages5 1 1 Revision Guide How FastGarret GordonNo ratings yet
- Chapter 16 (Kinetics)Document9 pagesChapter 16 (Kinetics)Richard KimNo ratings yet
- I. Answer The Following Questions: UNIT-7 Chemical KineticsDocument30 pagesI. Answer The Following Questions: UNIT-7 Chemical KineticsElias jesu packiamNo ratings yet
- AQA 18 KineticsDocument20 pagesAQA 18 Kineticsleonidas.wujieweiNo ratings yet
- Theory: Name: Onkar Pardeshi Roll - No: 12 P.R.N: 12011027 Batch-B1Document6 pagesTheory: Name: Onkar Pardeshi Roll - No: 12 P.R.N: 12011027 Batch-B1Onkar BhosleNo ratings yet
- Class Notes On KineticsDocument11 pagesClass Notes On KineticsjollyNo ratings yet
- PPG - Kinetika KimiaDocument42 pagesPPG - Kinetika KimiamuaffifahNo ratings yet
- 2.10 Zero-Order ReactionsDocument4 pages2.10 Zero-Order ReactionsDr-SabaJamilNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsManohar MaripeNo ratings yet
- Kubota 2001Document6 pagesKubota 2001Miguel AugustoNo ratings yet
- Hidrolisa Pati Dari Kulit Singkong (Variabel Ratio Bahan Dan Konsentrasi Asam)Document6 pagesHidrolisa Pati Dari Kulit Singkong (Variabel Ratio Bahan Dan Konsentrasi Asam)Af'idatun NissaNo ratings yet
- Exercise O-1 - Chemical Kinetics ExerciseDocument16 pagesExercise O-1 - Chemical Kinetics Exercisemishraanuj3011No ratings yet
- Ideal ReactorsDocument6 pagesIdeal ReactorsAnalyn C. BejasaNo ratings yet
- Alcohols ADocument4 pagesAlcohols AcarlitoNo ratings yet
- Estudio Cinetico de Transesterificacion de Acetato de Metilo Con Catalizador Con NbutiloDocument6 pagesEstudio Cinetico de Transesterificacion de Acetato de Metilo Con Catalizador Con NbutiloValeria Villanueva CervantesNo ratings yet
- 4.1.2, 4.1.3 Exam QuestionsDocument9 pages4.1.2, 4.1.3 Exam QuestionsHyder OmarNo ratings yet
- Cpo ReportDocument20 pagesCpo ReportSyeda Khaliqa HamidNo ratings yet
- B) From Hydrocarbon:-: A) by Free Radical Halogenation ReactionDocument2 pagesB) From Hydrocarbon:-: A) by Free Radical Halogenation ReactionRaju kumarNo ratings yet
- Reaction RatesDocument52 pagesReaction Ratestausman100% (1)
- Kinetic Analysis of Tyrosinase Enzyme: Experiment #5Document39 pagesKinetic Analysis of Tyrosinase Enzyme: Experiment #5CareyTranNo ratings yet
- Quiz 4.2Document3 pagesQuiz 4.2Annabelle Cebrero Paulo AlbaoNo ratings yet
- Catalyst HandBook - Second Edition - JMDocument290 pagesCatalyst HandBook - Second Edition - JMAhmed NagyNo ratings yet
- Biochemical Engineering Final RequirementDocument5 pagesBiochemical Engineering Final RequirementLeeGonzaLgoNo ratings yet
- 7.016 Recitation 3 - Fall 2018: (Note: The Recitation Summary Should NOT Be Regarded As The Substitute For Lectures)Document6 pages7.016 Recitation 3 - Fall 2018: (Note: The Recitation Summary Should NOT Be Regarded As The Substitute For Lectures)Manish SarkarNo ratings yet
- Robinson Annulation Reaction of 3-Nitrochalcone With Ethyl AcetoacetateDocument9 pagesRobinson Annulation Reaction of 3-Nitrochalcone With Ethyl AcetoacetateAmirul Azhar100% (5)
- 5C - Stoichiometry 3Document38 pages5C - Stoichiometry 3Vimanan A/L S. VelangganiNo ratings yet
- RPH Science ExperimentDocument5 pagesRPH Science Experiment⎝⏠⏝⏠⎠ Mohd Zaidi IsmailNo ratings yet
- Chem Kinet Meeting 2Document27 pagesChem Kinet Meeting 2Nuril AzmiNo ratings yet
- Rates of Reaction - Mini LabsDocument7 pagesRates of Reaction - Mini LabsPolly LeungNo ratings yet
- Enzyme Mech of ActionDocument50 pagesEnzyme Mech of ActionAmrit LalNo ratings yet
- Answer Key-H.w (2) - Oxidation Reduction Reaction-G11 PDFDocument3 pagesAnswer Key-H.w (2) - Oxidation Reduction Reaction-G11 PDFbedo lucyNo ratings yet
- 6 - Chemical Kinetics PDFDocument16 pages6 - Chemical Kinetics PDFthinkiit100% (1)
- Reagent Pentra C200: NO Nama Reagent Sampel Suhu Stabilitas Unopened Stabilitas After Opening Stabilitas Kalibrasi TestDocument1 pageReagent Pentra C200: NO Nama Reagent Sampel Suhu Stabilitas Unopened Stabilitas After Opening Stabilitas Kalibrasi TestAnonymous guHWicHNo ratings yet
- Mecanisme de ReactieDocument29 pagesMecanisme de ReactieEcaterina MoruzNo ratings yet
- CH 6 - Organic ReactionsDocument18 pagesCH 6 - Organic Reactionskevincai96No ratings yet
- Kinetic Methods of Analysis: Chapter 15 - 1Document9 pagesKinetic Methods of Analysis: Chapter 15 - 1natsdorfNo ratings yet
- 02-Chemical Kinetic - Telegram - @JEE - BOOKSDocument11 pages02-Chemical Kinetic - Telegram - @JEE - BOOKSRdNo ratings yet