Professional Documents
Culture Documents
Methods of Polymerization
Methods of Polymerization
Uploaded by
Jim LivingstonCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Methods of Polymerization
Methods of Polymerization
Uploaded by
Jim LivingstonCopyright:
Available Formats
Unit 2 2024
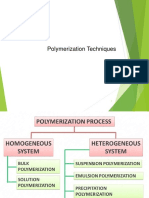
METHODS OF POLYMERIZATION
polymerisation is an exothermic reaction almost all commercial process of polymerization are
liquid phase reactions. The polymerisation process may be carried out in two systems :
(1) Homogeneous system 2) Heterogeneous system
(1) Homogeneous System
In this system, following types of polymerisation is possible:
(a) Bulk Polymerisation (b) Solution Polymerisation (c) Melt Polycondensation
(a) Bulk Polymerisation:
In this type of polymerization the monomer is taken in liquid form. The initiators, chain transfer
agent are dissolved in monomer. Now the system becomes homogenous. The reaction mass is
heated for the purpose of initiating the polymerization reaction and is kept under agitation for
proper heat and mass transfer. As the polymerization proceeds the viscosity of the medium
increases and mixing becomes difficult leading to products with very broad molecular weight
distribution. The reaction is purely exothermic.
The advantages of bulk polymerisation are the use of simple equipment, direct recovery of the
polymer and minimum chances of contamination. This technique of polymerisation is widely
used in the free-radical polymerisation of methyl methacrylate or styrene to get cast sheetings
and transparent moulding powder. It is also used to get polyvinyl chloride resin from vinyl
chloride.
Fig1: Bulk polymeriztion of styrene
SOLUTION POLYMERIZATION
Monomer is dissolved in suitable solvent along with chain transfer agents and initiators (free
radical initiator). Catalyst may be ionic or co-ordination catalyst which can either dissolved or
suspended. The presence of inert solvents medium helps to control viscosity, increase and
1 D.JIM LIVINGSTON| St. John’s College
Unit 2 2024
promote proper heat transfer. Process may be continuous/batch wise operation. The product
obtained is in liquid phase. For most cases water is taken as solvent.
The advantage of this process is that the presence of inert solvent medium helps to control
viscosity and promote a uniform heat transfer. Solution technique is used in industrial production
of polyacrylonitrile by free-radical polymerisation and also polyisobutylene by cationic
polymerisation. This technique is exclusively used in the production of block co-polymers.
Fig2: cationic polymerization of isobutylene
Melt Polycondensation
In this method, one of the monomers used is solid, which cannot decompose around its melting
point. The reaction has to be carried out in an inert atmosphere of N2 or CO2 to avoid side
reactions such as oxidation, decarboxylation, etc. Sometimes, the reaction is carried out under
reduced pressure to initiate the removal of the by product. The production of polyethylene
terephthalate from dimethyl terephthalate and ethylene glycol, and nylon 66 are prepared by
using melt polycondensation technique.
The major disadvantage of this technique is removal of the byproduct becomes extremely
difficult because there is much increase in the viscosity of the medium. The polymer formed is in
molten state at the reaction temperature and it is important to isolate it from the reactor during
hot condition, otherwise it will solidify inside the reactor and removal becomes very hard.
Fig3(a): schematic representation of melt
polycondensation (b)melt polycondensation of PTTA
2 D.JIM LIVINGSTON| St. John’s College
Unit 2 2024
Heterogeneous System
Heterogeneous system falls under two categories;
(a) Suspension Polymerisation (b) Emulsion Polymerisation
(a) Suspension Polymerisation
In this technique only water-insoluble monomers can be polymerised. The monomer molecules
are suspended in water and mixed with surface active agents and water-soluble protective
colloids. The initiators are monomer soluble. This is an economical process because here water is
taken as heat transfer medium and polymerisation proceeds to 100% conversion. The product is
obtained as spherical beads or pearls. For this reason, this technique is known as beads or pearl
polymerisation.
Isolation of the product is quite easy because this technique involves only filtration of the beads.
The washed and dried polymer products can be used as such for moulding, coating and adhesive
purposes. By suspension polymerisation method, polyvinyl acetate beads, polystyrene beads and
styrene-divinyl copolymer beads can be produced. A fairly narrow molecular weight product can
be achieved by this technique.
(b) Emulsion Polymerisation
In this system, the monomer is dispersed in the aqueous phase as a uniform emulsion, and the
emulsion is stabilised by surfactants (i.e., surface active agents), protective colloids and by
certain buffers. persulftes or hydrogen peroxide can be used as initiators. Redox initiators are
also widely used in this system. The surfactant molecule consists of two parts a long non-polar
hydrocarbon chain, and a polar group. In emulsifier molecule, the non-polar part attached with
polar end, and form micelle. The hydrocarbon ends of all the emulsifier come close to each other
at the interior and polar end align themselves outward. Emulsification can be achieved when the
monomer is added and agitated. In the emulsion system, a monomer droplets and micelles are
dispersed uniformly.
3 D.JIM LIVINGSTON| St. John’s College
Unit 2 2024
Fig 5: emulsion polymerization of styrene which
is used in thermocol preperation
In this process, the rate of polymerisation is very high, because the polymerisation is proceeded
at the micelles, where the surface/volume ratio is much large. Emulsion polymerisation is a most
widely used industrial technique. The monomers e.g., butadiene, chloroprene, vinyl acetate, vinyl
chloride, acrylates and methacrylates, etc., are used to polymerised by this method. A very high
molecular weight product can be achieved by this technique.
INTERFACIAL CONDENSATION POLYMERISATION
In this type of polymerisation, two different medium are taken and polymer is formed at the
point of interface between two phases. Here, one phase is an aqueous solvent and another phase
is an organic medium. The two solutions are thoroughly agitated, and thus formation of an
emulsion takes place. At the same time, the interface surface/volume ratio is increased and,
hence, both the rate and degree of polymerisation becomes too high. The formed polymer
precipitates out, isolated from the slurry and washed well.
The formation of polymer at the interface is a diffusion-controlled process, a very high molecular
weight polymer can be obtained by this technique. A typical example of interfacial condensation,
p-phenylene diamine is dissolved in water and terephtholoyl chloride is taken in chloroform or
carbon tetrachloride. When these two solutions are brought in contact with each other, the
diamine molecules diffuse into the organic phase and react with acid chloride to give polymides
at the interface which is precipitated out immediately.
Fig 6: (a) schematicrepresentation
of interface polymerization
(b) synthesis of a polymer in lab
(c) preparation of a polyamide
using interface polymeriztion
4 D.JIM LIVINGSTON| St. John’s College
You might also like
- Metal CarbonylsDocument12 pagesMetal CarbonylsJim Livingston57% (7)
- Experiment 1Document11 pagesExperiment 1Kelvin LimNo ratings yet
- Styrene BulkPolymerizationDocument2 pagesStyrene BulkPolymerizationNitin Hansalia100% (1)
- Experiment 1Document17 pagesExperiment 1Kelvin Lim100% (3)
- Artificial HeartDocument30 pagesArtificial HeartJim Livingston100% (2)
- SiliconesDocument29 pagesSiliconesJim Livingston100% (1)
- Unit 1 Techniques of PolymerizationDocument11 pagesUnit 1 Techniques of PolymerizationShevaniga SridharNo ratings yet
- Chem3020: Polymer Chemistry: Polymerization TechniquesDocument13 pagesChem3020: Polymer Chemistry: Polymerization TechniquesLuan Gabriel100% (1)
- Unit 2Document26 pagesUnit 2Pavi PaviNo ratings yet
- ICH404-Lecture Note 1Document2 pagesICH404-Lecture Note 1Lukman Bola Abdulra'ufNo ratings yet
- CHE 417 Lecture 8Document7 pagesCHE 417 Lecture 8Iteoluwakiishi AberuagbaNo ratings yet
- FBC Chem420-U1 Lec04Document20 pagesFBC Chem420-U1 Lec04tityjimmy6No ratings yet
- Polymer Class 2Document17 pagesPolymer Class 2Ñojib Ëasar ProttoyNo ratings yet
- M.F PolymerizationDocument14 pagesM.F Polymerizationsaqib sulmanNo ratings yet
- M.F PolymerizationDocument14 pagesM.F PolymerizationM. MoizNo ratings yet
- Plastic Industries: An OverviewDocument10 pagesPlastic Industries: An OverviewKubra ĖdrisNo ratings yet
- CHAPTER 3 Industrial Polymerization Reactions & TechniquesDocument30 pagesCHAPTER 3 Industrial Polymerization Reactions & Techniquesabdisahurisa24No ratings yet
- 2 Methods of PolymerizationDocument13 pages2 Methods of Polymerizationbt21102047 Vishwajeet YadavNo ratings yet
- Lecture Notes - Polymer Reaction EngineeringDocument8 pagesLecture Notes - Polymer Reaction EngineeringTenson SichoneNo ratings yet
- 1.4 Polymerization TechniquesDocument3 pages1.4 Polymerization TechniquesAnkit ParsoyaNo ratings yet
- Four Polymerization Techniques Bulk Solution Suspension and Emulsion PDFDocument6 pagesFour Polymerization Techniques Bulk Solution Suspension and Emulsion PDFGosa harikrishnaNo ratings yet
- Four Polymerization Techniques (Bulk, Solution, Suspension and Emulsion)Document6 pagesFour Polymerization Techniques (Bulk, Solution, Suspension and Emulsion)Ashokkumar Parthipan96% (48)
- 4 PolymerizationtechniquesDocument24 pages4 PolymerizationtechniquesMuzzamilNo ratings yet
- Unit 5 NotesDocument25 pagesUnit 5 NotesShubham DubeyNo ratings yet
- Solution and Bulk PolymerizationDocument7 pagesSolution and Bulk PolymerizationLuan GabrielNo ratings yet
- Polymerization TechniquesDocument24 pagesPolymerization Techniquesvalorantclips7.ioNo ratings yet
- Physical Properties of PmmaDocument17 pagesPhysical Properties of PmmaNaba ShahNo ratings yet
- Polymerisation TechniquesDocument9 pagesPolymerisation TechniquesNithishNo ratings yet
- 20 21 HK1 3rdDocument36 pages20 21 HK1 3rdHuy Ha0% (1)
- Aa PDFDocument7 pagesAa PDFnguyen duyNo ratings yet
- Scher 1998Document7 pagesScher 1998jesus.gerson.1996No ratings yet
- 2018 Liu Yang Self-Stabilized Precipitation PolymerizationDocument12 pages2018 Liu Yang Self-Stabilized Precipitation PolymerizationTay HuiaNo ratings yet
- Microspheres: Click To Edit Master Subtitle StyleDocument33 pagesMicrospheres: Click To Edit Master Subtitle StyleSwetha RanjithKumarNo ratings yet
- Polymerization TechniquesDocument24 pagesPolymerization TechniquesA.K.M. Rashedul IslamNo ratings yet
- Introduction To PolymerisationDocument40 pagesIntroduction To PolymerisationDHANANJAY RAJNIKANTBHAI BODANo ratings yet
- Chapter 3-1 PolymerDocument16 pagesChapter 3-1 PolymerAbel TayeNo ratings yet
- Polymerization TechniquesDocument4 pagesPolymerization TechniquesSyed Ali HaiderNo ratings yet
- Microencapsulation TechnologiesDocument26 pagesMicroencapsulation TechnologiesArezoo JamNo ratings yet
- Teoria Emulsion PolymerDocument7 pagesTeoria Emulsion PolymerSantos de PradosNo ratings yet
- Techniques of PolymerizationDocument8 pagesTechniques of PolymerizationMadhavanIceNo ratings yet
- VV 2-0 PDFDocument7 pagesVV 2-0 PDFA MahmoodNo ratings yet
- Polymerization TechniquesDocument24 pagesPolymerization TechniquesPaolo ScafettaNo ratings yet
- Industrial Polymer PPT With BDU TemplateDocument228 pagesIndustrial Polymer PPT With BDU Templateabdisahurisa24No ratings yet
- Polymer Class 3Document9 pagesPolymer Class 3Ñojib Ëasar ProttoyNo ratings yet
- Emulsion PolymerizationDocument8 pagesEmulsion PolymerizationRyan VasquezNo ratings yet
- MicroencapsulationDocument7 pagesMicroencapsulationRai WaqasNo ratings yet
- Styrene MonomerDocument13 pagesStyrene MonomerSerkan Gecim100% (2)
- 3 s2.0 B0080431526004939 MainDocument6 pages3 s2.0 B0080431526004939 MainMohammed JamaliNo ratings yet
- Project 1 - Different Techniques of A Polymerization Process With Real Industrial Practice - Group 2Document11 pagesProject 1 - Different Techniques of A Polymerization Process With Real Industrial Practice - Group 2Jonathan EthanNo ratings yet
- Preparation of NanoparticlesDocument54 pagesPreparation of NanoparticlesMahwish TariqNo ratings yet
- MicrospheresDocument48 pagesMicrospheresVouge Mode100% (2)
- Module 1Document19 pagesModule 1ABIGAIL OLAJUMOKE JOSEPHNo ratings yet
- Polystyrene Methods 2520of 2520production 0303Document6 pagesPolystyrene Methods 2520of 2520production 0303Anish KumarNo ratings yet
- Polymerisation Practice TEPEDocument39 pagesPolymerisation Practice TEPEDr. Dure Najaf Iqbal100% (3)
- 3 Rekayasa Reaksi PolimerisasiDocument86 pages3 Rekayasa Reaksi Polimerisasirudy_423522658No ratings yet
- Teknologi Polimerisasi - ShareDocument91 pagesTeknologi Polimerisasi - ShareAditya FaujiansyahNo ratings yet
- Polymerization of Acrylic EsterDocument7 pagesPolymerization of Acrylic EsterMichelleYapNo ratings yet
- Polyvinylchloride (PVC) : Eco-Profiles of The European Plastics IndustryDocument15 pagesPolyvinylchloride (PVC) : Eco-Profiles of The European Plastics IndustryGuillianNo ratings yet
- Bulk and Solution PolymerizationDocument3 pagesBulk and Solution PolymerizationAshokkumar ParthipanNo ratings yet
- Chapter 3 Polymerization TechDocument38 pagesChapter 3 Polymerization Techfyza8790No ratings yet
- 3 MicrospheresDocument48 pages3 MicrospheresFrooti SoujiNo ratings yet
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeFrom EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNo ratings yet
- Emulsion-based Systems for Delivery of Food Active Compounds: Formation, Application, Health and SafetyFrom EverandEmulsion-based Systems for Delivery of Food Active Compounds: Formation, Application, Health and SafetyShahin RoohinejadNo ratings yet
- Fajan's RuleDocument14 pagesFajan's RuleJim Livingston0% (1)
- Wastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryDocument22 pagesWastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryJim LivingstonNo ratings yet
- Carbanions and Substitution ReactionDocument11 pagesCarbanions and Substitution ReactionJim Livingston100% (2)
- Inorganic Chemistry: Atomic StructureDocument25 pagesInorganic Chemistry: Atomic StructureJim LivingstonNo ratings yet
- Surface Chemistry: AdsorptionDocument19 pagesSurface Chemistry: AdsorptionJim LivingstonNo ratings yet
- Waste Water TreatmentDocument10 pagesWaste Water TreatmentJim LivingstonNo ratings yet
- Introduction To Infrared Spectroscopy: D. Jim Livingston Faculty of Chemistry St. John'S CollegeDocument22 pagesIntroduction To Infrared Spectroscopy: D. Jim Livingston Faculty of Chemistry St. John'S CollegeJim LivingstonNo ratings yet
- D. Jim Livingston: Asst. Professor Department of Chemistry St. John's CollegeDocument12 pagesD. Jim Livingston: Asst. Professor Department of Chemistry St. John's CollegeJim LivingstonNo ratings yet
- Unit-2: D. Jim LivingstonDocument26 pagesUnit-2: D. Jim LivingstonJim Livingston100% (1)
- Inorganic Compounds in MedicinesDocument27 pagesInorganic Compounds in MedicinesJim LivingstonNo ratings yet
- Determination of Blood GlucoseDocument14 pagesDetermination of Blood GlucoseJim LivingstonNo ratings yet
- Metal CarbonylsDocument17 pagesMetal CarbonylsJim Livingston100% (3)
- Wastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryDocument30 pagesWastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryJim Livingston100% (1)
- Metal CarbonylsDocument17 pagesMetal CarbonylsJim Livingston100% (3)
- Metal NitrosylsDocument27 pagesMetal NitrosylsJim Livingston79% (14)
- Metal Carbonyls: D. Jim LivingstonDocument12 pagesMetal Carbonyls: D. Jim LivingstonJim Livingston100% (2)
- Nomenclature and 18 Electron RuleDocument25 pagesNomenclature and 18 Electron RuleJim Livingston100% (1)
- Summer Research ProjectDocument23 pagesSummer Research ProjectJim LivingstonNo ratings yet
- Organometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiDocument23 pagesOrganometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiJim LivingstonNo ratings yet
- Contact Lens: T.Johnson Francis Selvan Iii BSC Chemistry St. Johns CollegeDocument68 pagesContact Lens: T.Johnson Francis Selvan Iii BSC Chemistry St. Johns CollegeJim Livingston100% (1)
- Artificial Skin: P. Petchiammal Iii B.SC Chemistry, St. John'S CollegeDocument21 pagesArtificial Skin: P. Petchiammal Iii B.SC Chemistry, St. John'S CollegeJim LivingstonNo ratings yet
- Polymerization TechniquesDocument18 pagesPolymerization TechniquesJim LivingstonNo ratings yet
- Polymer Processing (Molding) : D. Jim Livingston Faculty of Chemistry, St. John's CollegeDocument26 pagesPolymer Processing (Molding) : D. Jim Livingston Faculty of Chemistry, St. John's CollegeJim LivingstonNo ratings yet
- 165-184 Parameters MCDocument20 pages165-184 Parameters MCluis miguel huarita castellonNo ratings yet
- Mapei Dam Cement Reducing Agents Technical NotebookDocument20 pagesMapei Dam Cement Reducing Agents Technical NotebookfaheemqcNo ratings yet
- PorosityDocument19 pagesPorosityRoland EndrészNo ratings yet
- Selen Iodometric enDocument4 pagesSelen Iodometric enSena KulaksızNo ratings yet
- h2s Scavenging BRDocument12 pagesh2s Scavenging BROmid LarkiNo ratings yet
- Biochemistry Virtual Laboratory Experiment: CarbohydratesDocument2 pagesBiochemistry Virtual Laboratory Experiment: CarbohydratesJuneth Billones100% (1)
- Chapter 15Document19 pagesChapter 15Misbahudin AlhanifNo ratings yet
- Chapter - 1 - Atoms Molecules StoichiometryDocument25 pagesChapter - 1 - Atoms Molecules StoichiometrylidiaepNo ratings yet
- Qenos Alkatane GF7740F2Document1 pageQenos Alkatane GF7740F2Manoj SahuNo ratings yet
- Precision Determination of Precious Metals With ICP-OESDocument25 pagesPrecision Determination of Precious Metals With ICP-OESAbdulrahman JradiNo ratings yet
- Duoline Best Practices Intervention & Chemical CompatabilityDocument5 pagesDuoline Best Practices Intervention & Chemical CompatabilityWISSAMSOULIMANNo ratings yet
- Reactions of Ar - Compds.21Document140 pagesReactions of Ar - Compds.21hamdy solimanNo ratings yet
- American Galvanizers Association-Hot-Di P Galvanizing vs. Paint in Life-Cycle Assessment PDFDocument4 pagesAmerican Galvanizers Association-Hot-Di P Galvanizing vs. Paint in Life-Cycle Assessment PDFdgkmurtiNo ratings yet
- PDF Document 3Document37 pagesPDF Document 3miriam harriottNo ratings yet
- RHEOLOGYDocument18 pagesRHEOLOGYArchie CabacheteNo ratings yet
- Analytical Method Validation NutraceuticalsDocument6 pagesAnalytical Method Validation NutraceuticalsBhaskar NapteNo ratings yet
- Non Aqueous SolventsDocument24 pagesNon Aqueous SolventsRSLNo ratings yet
- Chemical Examination and Insecticidal Properties of Tagetes Erecta and PDFDocument10 pagesChemical Examination and Insecticidal Properties of Tagetes Erecta and PDFAmit KumariNo ratings yet
- LI-7200RS and SmartFlux 2 User CalibrationDocument1 pageLI-7200RS and SmartFlux 2 User CalibrationTomás Arturo Soto MurilloNo ratings yet
- N063f ER Design of FasteningsDocument114 pagesN063f ER Design of FasteningsVlad YzuNo ratings yet
- Detailed Lesson Plan Science 5 WeatheringDocument23 pagesDetailed Lesson Plan Science 5 WeatheringJose, Jonnabelle IgnacioNo ratings yet
- Chemical Reactions and Equations: Multiple Choice QuestionsDocument8 pagesChemical Reactions and Equations: Multiple Choice QuestionsSahana karpagamNo ratings yet
- Electrochemistry Assignment-1Document2 pagesElectrochemistry Assignment-1Anubhav SinghNo ratings yet
- 12 - Evaluasi Shaly SandDocument31 pages12 - Evaluasi Shaly SandYordanNo ratings yet
- Guided Plan-5 (E)Document4 pagesGuided Plan-5 (E)abhiraw30062005No ratings yet
- MCE 541 - Cement Replacement Materials Pozzolanic ReactionsDocument46 pagesMCE 541 - Cement Replacement Materials Pozzolanic ReactionsatousazakariaeiNo ratings yet
- Improvement of Method For Determination of Isocyanate Group ContentDocument5 pagesImprovement of Method For Determination of Isocyanate Group ContentBUSTANUL RIZKY RIZKYNo ratings yet
- Chemistry Higher Level Paper 3: Instructions To CandidatesDocument32 pagesChemistry Higher Level Paper 3: Instructions To CandidatesJuan Camilo VargasNo ratings yet
- Luke Stainer Osmosis Practical Write UpDocument5 pagesLuke Stainer Osmosis Practical Write UpMaan PatelNo ratings yet
- Technical Data Sheet Chryso Resicrete 2115 - 6066 - 1367Document2 pagesTechnical Data Sheet Chryso Resicrete 2115 - 6066 - 1367binodNo ratings yet