Professional Documents
Culture Documents
Electrochemistry
Electrochemistry
Uploaded by
ms0207345Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemistry
Electrochemistry
Uploaded by
ms0207345Copyright:
Available Formats
CHAPTER- 02
Electrochemistry
Branch of chemistry that deals with the production of electricity and the energy
released during the spontaneous reaction and is used to carry out non
spontaneous reaction transformations.
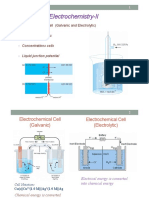
Electrochemical cell:
It is a device that converts chemical energy into electrical energy. These cells
are energy efficient and pollution free.
Galvanic or Voltaic cell:
The cell that converts chemical energy into electrical energy and has an
electrical potential equal to 1.1 V and the conc. Of the ions are unity.
Zn + Cu+2 → Zn+2 + Cu
Electrolytic cell:
A device that uses electrical energy to carry non spontaneous reaction chemical
reaction.
Application of external voltage in the Galvanic cell:
• Eext < 1.1 V
(i) Electrons flow from zinc to copper and current flows from copper
to zinc.
(ii) Zinc dissolves at anode and copper gets deposited at cathode.
• Eext = 1.1 V
(i) No flow of current and electrons hence no chemical reaction takes
place.
• Eext > 1.1 V
(i) It functions as an electrolytic cell hence, the electrons will flow in
the opposite direction i.e. from copper to zinc and current from
zinc to copper
(ii) Zinc gets deposited at cathode and copper dissolves at anode.
Half Cells or Redox Couple:
The two portions of the cell at which oxidation and reduction reactions takes place.
Electrode Potential:
Potential difference between the electrode and the electrolyte.
Standard Electrode Potential:
When the conc. of all the species involved in the half cell is unity then the
electrode potential is called standard electrode potential.
Cell Potential:
The potential difference between the two electrodes and is measured in volts.
E◦cell = E◦cathode - E◦anode
Electromotive Force (emf):
It is the cell potential difference between the electrode potential of the cathode
and anode.
Cell Representation:
A cell is represented by putting a vertical line between metal and electrolyte
solution and a double vertical line between the two electrolytes connected by
the salt bridge.
Eg. Cu + 2 Ag+ → Cu+2 + 2 Ag
For the above reaction the cell is represented as
Cu/Cu+2 Ag+ /Ag
Measurement of Electrode Potential:
Potential of an individual half cell cannot be measured. Thus acc.to convention,
a half cell called standard hydrogen potential (SHE)is set against the individual
half cell that has a zero potential at all temperatures and represented by
Pt(s) / H2 (g) / H+(aq)
SHE consist of platinum electrode coated with platinum black dipped in acid
solution and hydrogen gas is bubbled through it.
The conc. of both the reduced and oxidised form of hydrogen is unity, thus the
pressure of the gas is 1 bar and conc. of hydrogen ion is 1 molar.
SHE is taken as anode that has a electrode potential zero.
** Cu does not dissolve HCl as Cu has a positive value of standard electrode
potential thus Copper itself gets reduced than hydrogen.
** Metals like platinum, gold etc are used as inert electrode as they don’t
participate in the reaction but only provides its surface for the reaction to take
place.
** Electrochemical cells are extensively used for determining the pH of
solutions, solubility product, equilibrium constant and thermodynamic
properties and for potentiometric titration.
NERNST EQUATION:
This equation gave a relationship between the electrode potential , temperature
and the conc. of the metal ion.
M+(aq) + ne- → M(s)
E(M+/ M) = E◦(M+/ M) - RT ln [M] (i)
nF [M+]
where, R = 8.314 J/K/mol (gas constant)
T = 298 K (temperature)
F = 96500 C (faraday’s constt)
Substituting the values in eqn (i) we get,
Ecell = E◦cell - 0.0591 log [oxidation]
n [Reduction]
You might also like
- Experiment 5: Electrical Resistance and Ohm's LawDocument10 pagesExperiment 5: Electrical Resistance and Ohm's LawsyafNo ratings yet
- Unit 3 ElectrochemistryDocument14 pagesUnit 3 ElectrochemistrySuresh Dasaraddi100% (1)
- Electrochemistry &batteries 2018Document52 pagesElectrochemistry &batteries 2018santhoshNo ratings yet
- Unit 3 ElectrochemistryDocument51 pagesUnit 3 Electrochemistrysukaina fatimaNo ratings yet
- Notes Chem NewDocument17 pagesNotes Chem Newilias1973No ratings yet
- CHM 232 EMF of CellsDocument22 pagesCHM 232 EMF of Cellseadedeji247No ratings yet
- Electrochemical-Cells Kec PDFDocument10 pagesElectrochemical-Cells Kec PDFsachinNo ratings yet
- unit 1 electrode systemDocument15 pagesunit 1 electrode systemkarthikshetty97275No ratings yet
- Class 12 Chemistry Project (Electochemistry)Document10 pagesClass 12 Chemistry Project (Electochemistry)Raghvendra Pandey0% (1)
- ElectrochemistryDocument80 pagesElectrochemistryNitin NishantNo ratings yet
- Corrosion 4Document34 pagesCorrosion 4Faria Sultana MimiNo ratings yet
- Electro Analytical ChemistryDocument10 pagesElectro Analytical ChemistryStephany Solis GuerraNo ratings yet
- Electrochemistry Part 1Document35 pagesElectrochemistry Part 1ABHINAVNo ratings yet
- Lesson-3 ELECTROCHEMISTRY - 231017 - 181903Document12 pagesLesson-3 ELECTROCHEMISTRY - 231017 - 181903xyz1234cbaNo ratings yet
- Electochemistry PDFDocument29 pagesElectochemistry PDFAnshu KarmacharyaNo ratings yet
- SESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Document7 pagesSESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Ashok KumarNo ratings yet
- ElectrochemistryDocument20 pagesElectrochemistry나야No ratings yet
- Chem Unit 3Document29 pagesChem Unit 3dgoutham4926No ratings yet
- 3 Electro Chemistry 1Document40 pages3 Electro Chemistry 1Kalpana BidhanNo ratings yet
- 3 ElectroDocument19 pages3 ElectroRoxanneNo ratings yet
- Electrode PotentialDocument14 pagesElectrode PotentialVinay HaridasNo ratings yet
- Fuel CellDocument27 pagesFuel CellGallium TNo ratings yet
- Electrochemistry Theory EDocument30 pagesElectrochemistry Theory Ethinkiit100% (2)
- Electrochemical CellDocument30 pagesElectrochemical CellSubhu100% (1)
- Electrochemistry Notes by PradeepDocument6 pagesElectrochemistry Notes by PradeepPradeep Siddham50% (2)
- Electrochemistry FinalDocument58 pagesElectrochemistry Finalrp7workNo ratings yet
- Electrical Energy and Vice Versa. It Is Basically The Study ofDocument11 pagesElectrical Energy and Vice Versa. It Is Basically The Study ofJake WooNo ratings yet
- ElectrochemistryDocument17 pagesElectrochemistryAbhianv GuptaNo ratings yet
- Chapter 3 Electro ChemistryDocument20 pagesChapter 3 Electro ChemistryKritika MishraNo ratings yet
- Electrode PotentialDocument24 pagesElectrode PotentialZoeNo ratings yet
- Chap.1 ElectrochemistryDocument93 pagesChap.1 ElectrochemistryAnushkaSinhaNo ratings yet
- ElectroDocument13 pagesElectrodulalsushant3No ratings yet
- Principles of Electrochemistry: Potential & ThermodynamicsDocument13 pagesPrinciples of Electrochemistry: Potential & ThermodynamicsGonzalo AlmeidaNo ratings yet
- Department of Chemical EngineeringDocument12 pagesDepartment of Chemical EngineeringSheikh AliNo ratings yet
- M1 ElectrochemistryDocument11 pagesM1 ElectrochemistryMalvika RkNo ratings yet
- Conductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDocument12 pagesConductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDev Printing SolutionNo ratings yet
- Electrochemical Cells: Electrode PotentialDocument20 pagesElectrochemical Cells: Electrode PotentialMunazNo ratings yet
- Electro ChemistryDocument25 pagesElectro Chemistrytpvv sreenivasaraoNo ratings yet
- Lecture Notes - Engineering ChemistryDocument56 pagesLecture Notes - Engineering ChemistryadamjosephNo ratings yet
- NotesDocument20 pagesNotesP I X Ξ LNo ratings yet
- NotesDocument20 pagesNotesP I X Ξ LNo ratings yet
- Electrode ChemDocument17 pagesElectrode Chemapi-372366467% (3)
- Chapter One: Introduction of ElectrochemistryDocument29 pagesChapter One: Introduction of ElectrochemistryBayan O. Abu SaadaNo ratings yet
- Chemistry Notes For Class 12 Chapter 3 ElectrochemistryDocument17 pagesChemistry Notes For Class 12 Chapter 3 ElectrochemistryRavi Kumar50% (4)
- 202004021930364692ranvijay ElectrochemistryDocument11 pages202004021930364692ranvijay ElectrochemistryThantzinNo ratings yet
- ElectrochemistryDocument80 pagesElectrochemistrykunalwahNo ratings yet
- CLASS 12 - Chemistry - Notes - ch02 - ElectrochemistryDocument8 pagesCLASS 12 - Chemistry - Notes - ch02 - Electrochemistrynethan171106No ratings yet
- Course Material CO1Document19 pagesCourse Material CO1yaswanth kandulaNo ratings yet
- Electrochemical Energy Systems DV 2021 EvenDocument116 pagesElectrochemical Energy Systems DV 2021 EvenAditi BardhanNo ratings yet
- ElectrochemistryDocument80 pagesElectrochemistryAshish KumarNo ratings yet
- B. Electrochemistry 2 1Document37 pagesB. Electrochemistry 2 1Dank CoderNo ratings yet
- Unit 5: ElectrochemistryDocument14 pagesUnit 5: ElectrochemistryOGEGA KERUBONo ratings yet
- Notes ElectroDocument15 pagesNotes ElectrodasdwasdwadNo ratings yet
- Electrochemistry 20Document21 pagesElectrochemistry 20danishNo ratings yet
- MODULE 2-Electrode SystemDocument27 pagesMODULE 2-Electrode Systemchethansl 826No ratings yet
- Electrochemistry Short NotesDocument5 pagesElectrochemistry Short Notestukutanigel423No ratings yet
- 1 Energy SystemsDocument21 pages1 Energy Systemsadwar1807No ratings yet
- Essay On Hydrogen TechnologyDocument22 pagesEssay On Hydrogen TechnologyBruno EggertNo ratings yet
- Redox Lab Report CompleteDocument15 pagesRedox Lab Report CompleteJackson KasakuNo ratings yet
- Producing Hydrogen Through Electrolysis and Other ProcessesDocument50 pagesProducing Hydrogen Through Electrolysis and Other ProcessesAnedo 1909No ratings yet
- 5 Measurement of Industrial ParametersDocument32 pages5 Measurement of Industrial ParametersumarsaboNo ratings yet
- Chm221 Chapter 5Document42 pagesChm221 Chapter 5Badrudin JundailiNo ratings yet
- Water Level Indicator Mini Project ReportDocument22 pagesWater Level Indicator Mini Project Reportabhay yadavNo ratings yet
- كيمياء طبية8Document9 pagesكيمياء طبية8Moħämmễḑ ĪþräħễễmNo ratings yet
- ElectrochemistryDocument37 pagesElectrochemistryRichardNo ratings yet
- A. Conductor: Cat A - Module 3: Electrical FundamentalsDocument15 pagesA. Conductor: Cat A - Module 3: Electrical FundamentalsJohn MerahNo ratings yet
- Part - I: Subjective Questions: Section (A) : Galvanic Cell, Its Representation & Salt BridgeDocument28 pagesPart - I: Subjective Questions: Section (A) : Galvanic Cell, Its Representation & Salt BridgeGOURISH AGRAWALNo ratings yet
- OrganicInorganicChemistryTY2013 PDFDocument13 pagesOrganicInorganicChemistryTY2013 PDFSeafuddin ShikNo ratings yet
- Chapter6-Electrochemistry (Part 2)Document27 pagesChapter6-Electrochemistry (Part 2)Uswatun KhasanahNo ratings yet
- DET Series Earth Ground Electrode TestersDocument46 pagesDET Series Earth Ground Electrode TestersGaya HidupNo ratings yet
- Test 0N Redox EquilibriaDocument5 pagesTest 0N Redox EquilibriaLittle WizardNo ratings yet
- SL Paper3Document26 pagesSL Paper3Boshra NouriNo ratings yet
- Design and Fabrication of IoT Operated Cono WeederDocument7 pagesDesign and Fabrication of IoT Operated Cono WeederIJRASETPublicationsNo ratings yet
- ACT Thrissur Plus Two Easy QuestionsDocument23 pagesACT Thrissur Plus Two Easy QuestionsAbin Pm100% (1)
- EET100 Principles of Electrical Engineering 1Document89 pagesEET100 Principles of Electrical Engineering 1Filbert OmbongiNo ratings yet
- Kimia CHPTR 6Document138 pagesKimia CHPTR 6alisaNo ratings yet
- Yr 10 Chem Summer NoteDocument22 pagesYr 10 Chem Summer NoteTokoni DanielNo ratings yet
- Redox Reactions: Oxidation Is Loss Electrons (OIL)Document24 pagesRedox Reactions: Oxidation Is Loss Electrons (OIL)Night Mist7No ratings yet
- 2 Physical Chemistry 20nDocument210 pages2 Physical Chemistry 20nLaziNo ratings yet
- EDUC 3136 PSIII Chemistry PRACTICALS 2023 PDFDocument80 pagesEDUC 3136 PSIII Chemistry PRACTICALS 2023 PDFKgaugelo FenyaneNo ratings yet
- Report On Water Level Indicator With Alarm (1822228)Document16 pagesReport On Water Level Indicator With Alarm (1822228)Kajal MhetreNo ratings yet
- Prateek 1Document12 pagesPrateek 1Hayato ffNo ratings yet
- Chapter 1 Redox Equilibrium 1 StudentDocument80 pagesChapter 1 Redox Equilibrium 1 StudentJIanyun ChanNo ratings yet
- TPD ABCIL CAUSTIC PLANT MANUAL RevDocument42 pagesTPD ABCIL CAUSTIC PLANT MANUAL Revjavier pividoriNo ratings yet
- Final PDF For Chemistry Class XiiDocument172 pagesFinal PDF For Chemistry Class XiiSANJAY PARMARNo ratings yet
- Experiment No. 3 Construction of Aluminum Air BatteryDocument10 pagesExperiment No. 3 Construction of Aluminum Air BatteryknightruzelNo ratings yet