Professional Documents
Culture Documents
chm dpp 23
chm dpp 23
Uploaded by
notvivaan1106Copyright:
Available Formats
You might also like
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- ATP Star 2Document28 pagesATP Star 2Gowri ShankarNo ratings yet
- Stereoisomers Test Answers+ QuestionsDocument8 pagesStereoisomers Test Answers+ QuestionsNandakumar SNo ratings yet
- Target: Pre-Medical 2022: IsomerismDocument3 pagesTarget: Pre-Medical 2022: IsomerismankitrajjaindigitalNo ratings yet
- G.O.C & Isomerism - DPP 04 (Of Lec 07) - (Arjuna JEE 2023)Document3 pagesG.O.C & Isomerism - DPP 04 (Of Lec 07) - (Arjuna JEE 2023)jeemainsmaterial97No ratings yet
- Isomerism - Chem - NEET RRC PDFDocument7 pagesIsomerism - Chem - NEET RRC PDFsedida1374No ratings yet
- As Mhy FG 9 SVGy 5 M Qo KC2 oDocument52 pagesAs Mhy FG 9 SVGy 5 M Qo KC2 osingharyendra175No ratings yet
- Inorganic DPP PDFDocument3 pagesInorganic DPP PDFashutosh99878No ratings yet
- M-Caps-29: Chemistry: NEET & AIIMS 2018-19Document3 pagesM-Caps-29: Chemistry: NEET & AIIMS 2018-19Vishal SinghNo ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- PRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)Document5 pagesPRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)ABD 17No ratings yet
- IsomerismDocument2 pagesIsomerismricardobabu6t9No ratings yet
- Syll-3 11th Org Chem MTS...Document6 pagesSyll-3 11th Org Chem MTS...deveshjayakumaryadavNo ratings yet
- Optical IsomerismDocument3 pagesOptical IsomerismRaj RastogiNo ratings yet
- Isomerism - DPP-01 (Of Lecture 02) - Yakeen 3.0 2024Document3 pagesIsomerism - DPP-01 (Of Lecture 02) - Yakeen 3.0 2024bikrampaul021No ratings yet
- Isomerism DPP - With Solution PDFDocument20 pagesIsomerism DPP - With Solution PDFPiyush Agarwal50% (2)
- GOC - DPP 04 - Yakeen 2.0 2024 (Legend)Document3 pagesGOC - DPP 04 - Yakeen 2.0 2024 (Legend)bandarbarfilaNo ratings yet
- Stereoisomersm ALL ENTHUSE BATCHESDocument7 pagesStereoisomersm ALL ENTHUSE BATCHESTouseef AhmadNo ratings yet
- Ocd PP Special On Taut Omer Is MDocument3 pagesOcd PP Special On Taut Omer Is MKartik YadavNo ratings yet
- Surprise Test - OCDocument4 pagesSurprise Test - OCRajanikanta PriyadarshiNo ratings yet
- OC - Stereoisomerism - E - CSDocument36 pagesOC - Stereoisomerism - E - CSHARSHIT 12ANo ratings yet
- Stereochemistry of Organic Compounds MCQDocument17 pagesStereochemistry of Organic Compounds MCQShunmugasundaram ArunachalamNo ratings yet
- PRACTICE SHEET - 09 (Chemistry) : Basic Concept of Organic ChemistryDocument4 pagesPRACTICE SHEET - 09 (Chemistry) : Basic Concept of Organic ChemistryABD 17No ratings yet
- DPP # 13 Time: 30 Min.: 1. Column - I Column - IIDocument3 pagesDPP # 13 Time: 30 Min.: 1. Column - I Column - IIArjun SabnisNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- 659d28b7d925b30018265333 ## Isomerism Practice SheetDocument8 pages659d28b7d925b30018265333 ## Isomerism Practice Sheetabhishekrabidas94No ratings yet
- CT - Isomerism - Isomerism - 03042021 - Isomerism - Practice Sheet 1 To 4Document15 pagesCT - Isomerism - Isomerism - 03042021 - Isomerism - Practice Sheet 1 To 4Anita Akhilesh YadavNo ratings yet
- IsomerismDocument26 pagesIsomerismsharmaekta1801No ratings yet
- Isomerism: IndexDocument38 pagesIsomerism: IndexDEV UPPALNo ratings yet
- Isomerism Que1Document1 pageIsomerism Que1Ancient DebrisNo ratings yet
- ViewpdfDocument12 pagesViewpdfBhargav SinghaNo ratings yet
- Isomerism SKC Sir Unacademy QuestionDocument20 pagesIsomerism SKC Sir Unacademy Questionbanajeetchakraborty.18No ratings yet
- Nomenclature SheetDocument24 pagesNomenclature SheetAnknownNo ratings yet
- JEE Adv. Optical IsomerismDocument22 pagesJEE Adv. Optical IsomerismYuvarajNo ratings yet
- Syll-3 11th Org Chem MTS..Document7 pagesSyll-3 11th Org Chem MTS..deveshjayakumaryadavNo ratings yet
- Organic ChemDocument3 pagesOrganic Chemlinda.wairepoNo ratings yet
- Black Board Problems For JEE Advanced Set-7Document8 pagesBlack Board Problems For JEE Advanced Set-7DikshantNo ratings yet
- ExerciseDocument50 pagesExerciseAbhiNo ratings yet
- Chapter 6: Chirality: ProblemsDocument35 pagesChapter 6: Chirality: ProblemsAlejandra BriceñoNo ratings yet
- ISI R: Organic ChemistryDocument28 pagesISI R: Organic Chemistrysarvesh goyalNo ratings yet
- Isomerism Review 2Document10 pagesIsomerism Review 2ayesha sheikhNo ratings yet
- Strereoisomerism 92Document25 pagesStrereoisomerism 92Oshi JainNo ratings yet
- Compendium On Problems in Physical-Organic ChemistryDocument27 pagesCompendium On Problems in Physical-Organic ChemistryHaryokoe buzzNo ratings yet
- Isomerism - Practice Sheet - Lakshya JEE 2025Document21 pagesIsomerism - Practice Sheet - Lakshya JEE 2025Ishan Nautiyal 9 binsarNo ratings yet
- cabroxylic acidsDocument9 pagescabroxylic acidssrinivasnarne78No ratings yet
- Christ King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryDocument5 pagesChrist King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryajaybolarNo ratings yet
- 4.2.2 Polyesters and Polyamides MSDocument3 pages4.2.2 Polyesters and Polyamides MSpillboxsesame0sNo ratings yet
- Isomerism : DPPsDocument23 pagesIsomerism : DPPs7aayushsehgalNo ratings yet
- IIT-JAM 2016 With SolutionDocument25 pagesIIT-JAM 2016 With SolutiongauravNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- C1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesDocument84 pagesC1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesBlack InsomniaNo ratings yet
- General Organic Chemistry-03 - Assignments (New)Document22 pagesGeneral Organic Chemistry-03 - Assignments (New)Raju SinghNo ratings yet
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNo ratings yet
- Cooh Cooh H: H HC F CH BRDocument2 pagesCooh Cooh H: H HC F CH BRHarshNo ratings yet
- 0optical Isomerism - QuizDocument3 pages0optical Isomerism - QuizSanjay Mani Tripathi50% (2)
- ????? GOC DPP 02 Yakeen 2 0 2024 LegendDocument3 pages????? GOC DPP 02 Yakeen 2 0 2024 Legendsanjay.ranchi10No ratings yet
- 4.4, 4.5 TEST MS 1.: Observation StructureDocument4 pages4.4, 4.5 TEST MS 1.: Observation Structure3estherNo ratings yet
- e1e4b300ffc5fbe8f9d2830d555e0a4fDocument8 pagese1e4b300ffc5fbe8f9d2830d555e0a4fveenayaksachinsharmaNo ratings yet
- Class Test - Structural IsomersDocument3 pagesClass Test - Structural IsomersAlex SamNo ratings yet
- Scicent PPT 9 3 eDocument91 pagesScicent PPT 9 3 eApple LouNo ratings yet
- Synthesis of Iron Nanoparticles Using Peel ExtractDocument8 pagesSynthesis of Iron Nanoparticles Using Peel ExtractVũ Phi YếnNo ratings yet
- 02 Analytical Profiles of Drug Substances, Vol 02Document571 pages02 Analytical Profiles of Drug Substances, Vol 02nur syaraNo ratings yet
- NBS 25-1 Картки Стандарт Зразкiв Рентген-дифракцii 1962Document64 pagesNBS 25-1 Картки Стандарт Зразкiв Рентген-дифракцii 1962CementarNo ratings yet
- Gamazyme 700FN 12KGDocument15 pagesGamazyme 700FN 12KGA R Ahmed Razvi100% (1)
- UrineDocument13 pagesUrineShubham PatwaNo ratings yet
- General Chemistry 1 Activity Sheet Quarter 2 - MELC 4 Week 7Document14 pagesGeneral Chemistry 1 Activity Sheet Quarter 2 - MELC 4 Week 7LayNo ratings yet
- Polymers: Natural Rubber Latex Foam Reinforced With Micro-And Nanofibrillated Cellulose Via Dunlop MethodDocument16 pagesPolymers: Natural Rubber Latex Foam Reinforced With Micro-And Nanofibrillated Cellulose Via Dunlop MethodRini Ananda SiagianNo ratings yet
- Mintekbulletin 148Document4 pagesMintekbulletin 148mushava nyokaNo ratings yet
- Namma Kalvi 1 Chemistry Book Back One Mark Question Vol 1 216585Document14 pagesNamma Kalvi 1 Chemistry Book Back One Mark Question Vol 1 216585Cook with thirudargalNo ratings yet
- Making Crystals With SaltDocument2 pagesMaking Crystals With SaltNadia BasherNo ratings yet
- Salt Analysis General ProcedureDocument7 pagesSalt Analysis General Procedurefranklin mahizhaNo ratings yet
- Comparison, Similarities, Differences, Merits and Demrits of Methods of Effective Electrode Resistance ReductionDocument5 pagesComparison, Similarities, Differences, Merits and Demrits of Methods of Effective Electrode Resistance ReductionInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Preparation of AzomethineDocument4 pagesPreparation of Azomethinepranjal JaiswalNo ratings yet
- Amines - D27 Nov 2019Document5 pagesAmines - D27 Nov 2019Tr Mazhar PunjabiNo ratings yet
- Article 1 PDFDocument19 pagesArticle 1 PDFAnonymous 5bgTK7qzsNo ratings yet
- Alcohol, Phenol and EthersDocument5 pagesAlcohol, Phenol and Ethersyeet buoyNo ratings yet
- Chemistry ProjectDocument18 pagesChemistry ProjectMd RehanNo ratings yet
- Hardness: The Most Important Dates in The History of Hardness Testing Are As FollowsDocument4 pagesHardness: The Most Important Dates in The History of Hardness Testing Are As FollowsSanjay YadavNo ratings yet
- Chapter 1, PPT, NewDocument14 pagesChapter 1, PPT, Newyonatanteshome48No ratings yet
- LithographyDocument12 pagesLithographyاحمد سعد كاطعNo ratings yet
- Msds Sodium Cyanide SolutionDocument14 pagesMsds Sodium Cyanide SolutionThirugnanam NNo ratings yet
- Chapter 15 - Coatings-1Document32 pagesChapter 15 - Coatings-1Sumit Ghosh KabboNo ratings yet
- AE8009 Airframe Maintenance & RepairDocument24 pagesAE8009 Airframe Maintenance & RepairAeronaughtycs Hamdan0% (2)
- Chemical Oxygen Demand (COD) (Closed Reflux Method)Document8 pagesChemical Oxygen Demand (COD) (Closed Reflux Method)hayder alali100% (1)
- Chapter - 2 O G S S: Verview of RID Tiffened TructureDocument12 pagesChapter - 2 O G S S: Verview of RID Tiffened TructureJohnson AnthonyNo ratings yet
- Biologi Molekuler - Uniport, Symport, AntiportDocument2 pagesBiologi Molekuler - Uniport, Symport, AntiportCinsy PaskalineNo ratings yet
- ChemicalEngineering Che December-2023Document44 pagesChemicalEngineering Che December-2023Antonio Mungioli100% (1)
- 1.2 Assessed HomeworkDocument8 pages1.2 Assessed HomeworkNavine NavNo ratings yet
- Investigatory Project On Metal CouplingDocument24 pagesInvestigatory Project On Metal CouplingDude LukaazNo ratings yet
chm dpp 23
chm dpp 23
Uploaded by
notvivaan1106Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
chm dpp 23
chm dpp 23
Uploaded by
notvivaan1106Copyright:
Available Formats
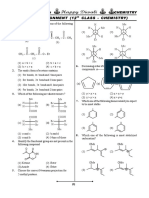
ISOMERISM
1. CH3CH2CH(CHO)CH2CH3 and CH3CH(CHO) 7. How many alkenes (only structural isomers) are
CH2CH 2CH3 are :- possible with the molecular formula C4H8
(1) Chain isomer (2) Positon isomer
(1) 4 (2) 3 (3) 5 (4) 6
(3) Metamerism (4) Functional isomer
2. Find out relation between the following 8. Which of the following is correct :-
compounds: O O
O COOH (1) ,
H
(1) O and Functional group isomers
O (2) ; Position isomers
O C–Cl
Cl
(2) and O
O
(3) ;
NH2 H2N H
(3) CH3CH2CH2CHCH3 and CH3CH2CHCH2CH3
COOH COOH Position and functional group isomers
(4) All of these
O O
9. Which of the following compounds can show
(4) O and geometrical isomerism :
O
(i) Pent-1-ene
3. How many amines are possible with the (ii) 1-chloropropene
molecular formula C4H11N (structural isomers (iii) 2-Methylbut-2-ene
only) :-
(iv) CH3–CH=CH–CH3
(1) 6 (2) 7 (3) 8 (4) 9
4. How many benzenoid isomers are possible for H H
molecular formula C7H8O :- Cl Cl
(1) 3 (2) 4 (3) 5 (4) 6 (v) C C (vi)
5. The relation between given compound is :- H H COOH COOH
H CH3
N CH3 N
(vii)
(1) Chain isomerism 10. Assign the configuration E/Z to the following
(2) Position isomerism compounds.
(3) Functional isomerism
(4) Metamerism H2N CH3 H2N OCH3
6. The structure below are :- C C
(i) (ii)
H C C
CH 3
H H H H Cl NO2 HS-CH2 F
H CH3 H CH3
H CH3 F Cl
C
(1) Not isomers (iii) (iv)
(2) Conformational isomers C
H D
(3) Cis-trans isomers
(4) Structural isomers
E Your Target is to secure Good Rank in Pre-Medical 2022 3
11. Which of the following compounds have non zero 13. Which is true for the following isomeric forms
dipole moment. I & II respectively :
(1) CCl4 CH3 CH3 CH3
H
C=C C=C
H CH3 H H
CH3 H
(2) C=C (I) (II)
H C2 H 5
Dipole Boiling Melting Stability
Cl H moment point point
(3) C=C
H Cl (1) I > II I > II II > I I > II
(2) II > I II > I II > I II > I
(3) I > II I > II I > II I > II
(4) Cl Cl
(4) II > I II > I I > II I > II
14. In which compound cis-trans nomenclature
12. Which one of the following is a Z isomer ? cannot be used :
CH3 Br (1) CH3–CH=CH–COOH
(1) C=C
(2) C6H5–CH=CH–C6H5
H Cl
(3) C6H5–CH=CHD
CH3 Cl
(2) C=C CH3
H Br (4) C6H5–CH=C
Cl
Cl 15. Compare dipole moment of the following
CH3
(3) C=C
Br H Cl Cl H Cl
(A) C=C ; C=C
H H Cl H
CH3 H
(4) C=C
H CH3 F CH3 F H
(B) C=C ; C=C
H H H CH3
(C) CH3–CH2–CCH ; CH3–CC–CH3
ANSWER KEY
Q ue. 1 3 4 5 6 7 8 11 12 13 14
Ans . 1 3 3 3 4 2 2 2 1 4 4
2. (i) Functional Group Isomerism 9. (ii), (iv), (v), (vi), (vii)
(ii) Functional Group Isomerism 10. (i) Z (ii) Z (iii) Z (iv) 2Z, 4E
(iii)Chain Isomerism 15. (A) i > ii (B) ii > i (C) i > ii
(iv)Functional Isomerism
4 Your Target is to secure Good Rank in Pre-Medical 2022 E
You might also like
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- ATP Star 2Document28 pagesATP Star 2Gowri ShankarNo ratings yet
- Stereoisomers Test Answers+ QuestionsDocument8 pagesStereoisomers Test Answers+ QuestionsNandakumar SNo ratings yet
- Target: Pre-Medical 2022: IsomerismDocument3 pagesTarget: Pre-Medical 2022: IsomerismankitrajjaindigitalNo ratings yet
- G.O.C & Isomerism - DPP 04 (Of Lec 07) - (Arjuna JEE 2023)Document3 pagesG.O.C & Isomerism - DPP 04 (Of Lec 07) - (Arjuna JEE 2023)jeemainsmaterial97No ratings yet
- Isomerism - Chem - NEET RRC PDFDocument7 pagesIsomerism - Chem - NEET RRC PDFsedida1374No ratings yet
- As Mhy FG 9 SVGy 5 M Qo KC2 oDocument52 pagesAs Mhy FG 9 SVGy 5 M Qo KC2 osingharyendra175No ratings yet
- Inorganic DPP PDFDocument3 pagesInorganic DPP PDFashutosh99878No ratings yet
- M-Caps-29: Chemistry: NEET & AIIMS 2018-19Document3 pagesM-Caps-29: Chemistry: NEET & AIIMS 2018-19Vishal SinghNo ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- PRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)Document5 pagesPRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)ABD 17No ratings yet
- IsomerismDocument2 pagesIsomerismricardobabu6t9No ratings yet
- Syll-3 11th Org Chem MTS...Document6 pagesSyll-3 11th Org Chem MTS...deveshjayakumaryadavNo ratings yet
- Optical IsomerismDocument3 pagesOptical IsomerismRaj RastogiNo ratings yet
- Isomerism - DPP-01 (Of Lecture 02) - Yakeen 3.0 2024Document3 pagesIsomerism - DPP-01 (Of Lecture 02) - Yakeen 3.0 2024bikrampaul021No ratings yet
- Isomerism DPP - With Solution PDFDocument20 pagesIsomerism DPP - With Solution PDFPiyush Agarwal50% (2)
- GOC - DPP 04 - Yakeen 2.0 2024 (Legend)Document3 pagesGOC - DPP 04 - Yakeen 2.0 2024 (Legend)bandarbarfilaNo ratings yet
- Stereoisomersm ALL ENTHUSE BATCHESDocument7 pagesStereoisomersm ALL ENTHUSE BATCHESTouseef AhmadNo ratings yet
- Ocd PP Special On Taut Omer Is MDocument3 pagesOcd PP Special On Taut Omer Is MKartik YadavNo ratings yet
- Surprise Test - OCDocument4 pagesSurprise Test - OCRajanikanta PriyadarshiNo ratings yet
- OC - Stereoisomerism - E - CSDocument36 pagesOC - Stereoisomerism - E - CSHARSHIT 12ANo ratings yet
- Stereochemistry of Organic Compounds MCQDocument17 pagesStereochemistry of Organic Compounds MCQShunmugasundaram ArunachalamNo ratings yet
- PRACTICE SHEET - 09 (Chemistry) : Basic Concept of Organic ChemistryDocument4 pagesPRACTICE SHEET - 09 (Chemistry) : Basic Concept of Organic ChemistryABD 17No ratings yet
- DPP # 13 Time: 30 Min.: 1. Column - I Column - IIDocument3 pagesDPP # 13 Time: 30 Min.: 1. Column - I Column - IIArjun SabnisNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- 659d28b7d925b30018265333 ## Isomerism Practice SheetDocument8 pages659d28b7d925b30018265333 ## Isomerism Practice Sheetabhishekrabidas94No ratings yet
- CT - Isomerism - Isomerism - 03042021 - Isomerism - Practice Sheet 1 To 4Document15 pagesCT - Isomerism - Isomerism - 03042021 - Isomerism - Practice Sheet 1 To 4Anita Akhilesh YadavNo ratings yet
- IsomerismDocument26 pagesIsomerismsharmaekta1801No ratings yet
- Isomerism: IndexDocument38 pagesIsomerism: IndexDEV UPPALNo ratings yet
- Isomerism Que1Document1 pageIsomerism Que1Ancient DebrisNo ratings yet
- ViewpdfDocument12 pagesViewpdfBhargav SinghaNo ratings yet
- Isomerism SKC Sir Unacademy QuestionDocument20 pagesIsomerism SKC Sir Unacademy Questionbanajeetchakraborty.18No ratings yet
- Nomenclature SheetDocument24 pagesNomenclature SheetAnknownNo ratings yet
- JEE Adv. Optical IsomerismDocument22 pagesJEE Adv. Optical IsomerismYuvarajNo ratings yet
- Syll-3 11th Org Chem MTS..Document7 pagesSyll-3 11th Org Chem MTS..deveshjayakumaryadavNo ratings yet
- Organic ChemDocument3 pagesOrganic Chemlinda.wairepoNo ratings yet
- Black Board Problems For JEE Advanced Set-7Document8 pagesBlack Board Problems For JEE Advanced Set-7DikshantNo ratings yet
- ExerciseDocument50 pagesExerciseAbhiNo ratings yet
- Chapter 6: Chirality: ProblemsDocument35 pagesChapter 6: Chirality: ProblemsAlejandra BriceñoNo ratings yet
- ISI R: Organic ChemistryDocument28 pagesISI R: Organic Chemistrysarvesh goyalNo ratings yet
- Isomerism Review 2Document10 pagesIsomerism Review 2ayesha sheikhNo ratings yet
- Strereoisomerism 92Document25 pagesStrereoisomerism 92Oshi JainNo ratings yet
- Compendium On Problems in Physical-Organic ChemistryDocument27 pagesCompendium On Problems in Physical-Organic ChemistryHaryokoe buzzNo ratings yet
- Isomerism - Practice Sheet - Lakshya JEE 2025Document21 pagesIsomerism - Practice Sheet - Lakshya JEE 2025Ishan Nautiyal 9 binsarNo ratings yet
- cabroxylic acidsDocument9 pagescabroxylic acidssrinivasnarne78No ratings yet
- Christ King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryDocument5 pagesChrist King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryajaybolarNo ratings yet
- 4.2.2 Polyesters and Polyamides MSDocument3 pages4.2.2 Polyesters and Polyamides MSpillboxsesame0sNo ratings yet
- Isomerism : DPPsDocument23 pagesIsomerism : DPPs7aayushsehgalNo ratings yet
- IIT-JAM 2016 With SolutionDocument25 pagesIIT-JAM 2016 With SolutiongauravNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- C1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesDocument84 pagesC1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesBlack InsomniaNo ratings yet
- General Organic Chemistry-03 - Assignments (New)Document22 pagesGeneral Organic Chemistry-03 - Assignments (New)Raju SinghNo ratings yet
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNo ratings yet
- Cooh Cooh H: H HC F CH BRDocument2 pagesCooh Cooh H: H HC F CH BRHarshNo ratings yet
- 0optical Isomerism - QuizDocument3 pages0optical Isomerism - QuizSanjay Mani Tripathi50% (2)
- ????? GOC DPP 02 Yakeen 2 0 2024 LegendDocument3 pages????? GOC DPP 02 Yakeen 2 0 2024 Legendsanjay.ranchi10No ratings yet
- 4.4, 4.5 TEST MS 1.: Observation StructureDocument4 pages4.4, 4.5 TEST MS 1.: Observation Structure3estherNo ratings yet
- e1e4b300ffc5fbe8f9d2830d555e0a4fDocument8 pagese1e4b300ffc5fbe8f9d2830d555e0a4fveenayaksachinsharmaNo ratings yet
- Class Test - Structural IsomersDocument3 pagesClass Test - Structural IsomersAlex SamNo ratings yet
- Scicent PPT 9 3 eDocument91 pagesScicent PPT 9 3 eApple LouNo ratings yet
- Synthesis of Iron Nanoparticles Using Peel ExtractDocument8 pagesSynthesis of Iron Nanoparticles Using Peel ExtractVũ Phi YếnNo ratings yet
- 02 Analytical Profiles of Drug Substances, Vol 02Document571 pages02 Analytical Profiles of Drug Substances, Vol 02nur syaraNo ratings yet
- NBS 25-1 Картки Стандарт Зразкiв Рентген-дифракцii 1962Document64 pagesNBS 25-1 Картки Стандарт Зразкiв Рентген-дифракцii 1962CementarNo ratings yet
- Gamazyme 700FN 12KGDocument15 pagesGamazyme 700FN 12KGA R Ahmed Razvi100% (1)
- UrineDocument13 pagesUrineShubham PatwaNo ratings yet
- General Chemistry 1 Activity Sheet Quarter 2 - MELC 4 Week 7Document14 pagesGeneral Chemistry 1 Activity Sheet Quarter 2 - MELC 4 Week 7LayNo ratings yet
- Polymers: Natural Rubber Latex Foam Reinforced With Micro-And Nanofibrillated Cellulose Via Dunlop MethodDocument16 pagesPolymers: Natural Rubber Latex Foam Reinforced With Micro-And Nanofibrillated Cellulose Via Dunlop MethodRini Ananda SiagianNo ratings yet
- Mintekbulletin 148Document4 pagesMintekbulletin 148mushava nyokaNo ratings yet
- Namma Kalvi 1 Chemistry Book Back One Mark Question Vol 1 216585Document14 pagesNamma Kalvi 1 Chemistry Book Back One Mark Question Vol 1 216585Cook with thirudargalNo ratings yet
- Making Crystals With SaltDocument2 pagesMaking Crystals With SaltNadia BasherNo ratings yet
- Salt Analysis General ProcedureDocument7 pagesSalt Analysis General Procedurefranklin mahizhaNo ratings yet
- Comparison, Similarities, Differences, Merits and Demrits of Methods of Effective Electrode Resistance ReductionDocument5 pagesComparison, Similarities, Differences, Merits and Demrits of Methods of Effective Electrode Resistance ReductionInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Preparation of AzomethineDocument4 pagesPreparation of Azomethinepranjal JaiswalNo ratings yet
- Amines - D27 Nov 2019Document5 pagesAmines - D27 Nov 2019Tr Mazhar PunjabiNo ratings yet
- Article 1 PDFDocument19 pagesArticle 1 PDFAnonymous 5bgTK7qzsNo ratings yet
- Alcohol, Phenol and EthersDocument5 pagesAlcohol, Phenol and Ethersyeet buoyNo ratings yet
- Chemistry ProjectDocument18 pagesChemistry ProjectMd RehanNo ratings yet
- Hardness: The Most Important Dates in The History of Hardness Testing Are As FollowsDocument4 pagesHardness: The Most Important Dates in The History of Hardness Testing Are As FollowsSanjay YadavNo ratings yet
- Chapter 1, PPT, NewDocument14 pagesChapter 1, PPT, Newyonatanteshome48No ratings yet
- LithographyDocument12 pagesLithographyاحمد سعد كاطعNo ratings yet
- Msds Sodium Cyanide SolutionDocument14 pagesMsds Sodium Cyanide SolutionThirugnanam NNo ratings yet
- Chapter 15 - Coatings-1Document32 pagesChapter 15 - Coatings-1Sumit Ghosh KabboNo ratings yet
- AE8009 Airframe Maintenance & RepairDocument24 pagesAE8009 Airframe Maintenance & RepairAeronaughtycs Hamdan0% (2)
- Chemical Oxygen Demand (COD) (Closed Reflux Method)Document8 pagesChemical Oxygen Demand (COD) (Closed Reflux Method)hayder alali100% (1)
- Chapter - 2 O G S S: Verview of RID Tiffened TructureDocument12 pagesChapter - 2 O G S S: Verview of RID Tiffened TructureJohnson AnthonyNo ratings yet
- Biologi Molekuler - Uniport, Symport, AntiportDocument2 pagesBiologi Molekuler - Uniport, Symport, AntiportCinsy PaskalineNo ratings yet
- ChemicalEngineering Che December-2023Document44 pagesChemicalEngineering Che December-2023Antonio Mungioli100% (1)
- 1.2 Assessed HomeworkDocument8 pages1.2 Assessed HomeworkNavine NavNo ratings yet
- Investigatory Project On Metal CouplingDocument24 pagesInvestigatory Project On Metal CouplingDude LukaazNo ratings yet