Professional Documents
Culture Documents
Raw data for Expt. 3
Raw data for Expt. 3
Uploaded by
tsun long TangCopyright:
Available Formats
You might also like
- PAG 11.2 - Acid Base Titration Curves GraphsDocument1 pagePAG 11.2 - Acid Base Titration Curves GraphsD ZooNo ratings yet
- Disassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide TitrationDocument19 pagesDisassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide Titrationwani280475% (4)
- Lab 15 - Acid-Base TitrationDocument5 pagesLab 15 - Acid-Base TitrationMelnykNo ratings yet
- Experiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Document15 pagesExperiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Noor Nasuha Noor Ariffin100% (1)
- CAPE - Chemistry LabsDocument19 pagesCAPE - Chemistry Labskelliann george100% (3)
- Graph of PH Against Volume of Naoh Titrated With Ch3Cooh: Equivalent Point: 29.20Document2 pagesGraph of PH Against Volume of Naoh Titrated With Ch3Cooh: Equivalent Point: 29.20adillaanisNo ratings yet
- PH and Buffer LabDocument13 pagesPH and Buffer LabAdellaine Lois GreyNo ratings yet
- CH Lab 3Document8 pagesCH Lab 3Mohammad Fahim NurNo ratings yet
- CH Lab 6Document7 pagesCH Lab 6Mohammad Fahim NurNo ratings yet
- Lab ReportDocument9 pagesLab ReportEssielve BatistilNo ratings yet
- Post-Lab Exer 1 PDFDocument5 pagesPost-Lab Exer 1 PDFDaniel Seth AndalNo ratings yet
- Lab ReportDocument6 pagesLab ReportShashaNo ratings yet
- Lab Report 1Document27 pagesLab Report 1szulkipeliNo ratings yet
- Lab 2 3Document11 pagesLab 2 3api-347340507No ratings yet
- Universiti Tunku Abdul Rahman Faculty of Science Bachelor of Science (Hons) ChemistryDocument6 pagesUniversiti Tunku Abdul Rahman Faculty of Science Bachelor of Science (Hons) ChemistryKirthinee JegatheesanNo ratings yet
- PH Against Volume Added (0.05M Hoac)Document4 pagesPH Against Volume Added (0.05M Hoac)Leroy ChengNo ratings yet
- CHM204 - Lab Report 2Document11 pagesCHM204 - Lab Report 2Romy MansourNo ratings yet
- Marcet Boiler Lab ReportDocument4 pagesMarcet Boiler Lab ReportJohnConor95% (41)
- Chem 2 Weak Base Strong Acid Lab ReportDocument6 pagesChem 2 Weak Base Strong Acid Lab ReportMohammad Izadi100% (1)
- Chem300Quiz 2ChEA LibutlibutDocument15 pagesChem300Quiz 2ChEA LibutlibutEredson LibutlibutNo ratings yet
- Exer 2 Post-Lab ReportDocument6 pagesExer 2 Post-Lab ReportKin DemoticaNo ratings yet
- Perhitungan Laboratorium-1Document11 pagesPerhitungan Laboratorium-1Akbar HidayatallahNo ratings yet
- Chemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaDocument18 pagesChemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaSita kumarNo ratings yet
- Lab 2 - (Titration)Document11 pagesLab 2 - (Titration)api-383698554No ratings yet
- Experiment 8Document5 pagesExperiment 88005Girish Kumar DasNo ratings yet
- Che485 - Exp 1Document21 pagesChe485 - Exp 1raisshakim02No ratings yet
- Neutralizacion NaOHDocument2 pagesNeutralizacion NaOHDarwin Laqui TiconaNo ratings yet
- AP CHEM Lab Acid Base Titration PDFDocument4 pagesAP CHEM Lab Acid Base Titration PDFMelnykNo ratings yet
- Preparation of PH Buffer SolutionsDocument5 pagesPreparation of PH Buffer SolutionscscsscNo ratings yet
- V Naoh (ML) PH: Otentiometric ItrationDocument9 pagesV Naoh (ML) PH: Otentiometric ItrationradyjrNo ratings yet
- CHEM Experiment 7Document11 pagesCHEM Experiment 7Mary Gencel EstrellaNo ratings yet
- Preparation of PH Buffer SolutionsDocument5 pagesPreparation of PH Buffer SolutionsAndrew Sumaya AldeNo ratings yet
- Buffer SolutionDocument8 pagesBuffer SolutionAbubakar UmarNo ratings yet
- Lab Report BoiDocument7 pagesLab Report BoiNORHIDAYATI BINTI MD GHAZALI MoeNo ratings yet
- Su AlbuDocument7 pagesSu AlbuLama SalahatNo ratings yet
- DkaDocument4 pagesDkaAlif Alfarisyi SyahNo ratings yet
- Tabla de Datos Procesados EVI Biología FinalDocument20 pagesTabla de Datos Procesados EVI Biología FinalYovanni SanchézNo ratings yet
- Chart TitleDocument4 pagesChart TitleBrigitte PorezNo ratings yet
- CHAPTER III AnachemDocument4 pagesCHAPTER III AnachemNur-Zhiana MuhiddiniNo ratings yet
- Chart Title: PH HCL PH Naoh 1 2 3 4 5 6 7 8 9 10 15 20 25 ML Adiciona DosDocument3 pagesChart Title: PH HCL PH Naoh 1 2 3 4 5 6 7 8 9 10 15 20 25 ML Adiciona DosSara Cicero RodriguezNo ratings yet
- Post Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Document7 pagesPost Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Mohammad Fahim NurNo ratings yet
- Phyical Lab CurvesDocument9 pagesPhyical Lab Curvescyrilbas16No ratings yet
- Estandarizacion NaohDocument10 pagesEstandarizacion NaohValeNo ratings yet
- Buffers: Lab ReportDocument8 pagesBuffers: Lab ReportjahangeerNo ratings yet
- CM 1502 Lab 3Document16 pagesCM 1502 Lab 3i000yearsNo ratings yet
- Estimation of Active IngredientDocument5 pagesEstimation of Active IngredientSuleman QaiserNo ratings yet
- Water Lab ReportDocument8 pagesWater Lab ReportMonica GellerNo ratings yet
- Datos Potenciometria 25-05-23Document6 pagesDatos Potenciometria 25-05-23Angela VillegasNo ratings yet
- Lab Report 3 KotDocument15 pagesLab Report 3 KotNikMuhammadIzzatNo ratings yet
- CAPE - Chemistry LabsDocument19 pagesCAPE - Chemistry Labskelliann georgeNo ratings yet
- Experiment 2Document6 pagesExperiment 2Sinichi IzumiNo ratings yet
- Determination-Of-Freezing-Point-DepressionDocument8 pagesDetermination-Of-Freezing-Point-DepressionAllan TampusNo ratings yet
- Chemical Reaction Engineering Laboratory: Title of Experiment: Production of CODocument10 pagesChemical Reaction Engineering Laboratory: Title of Experiment: Production of COHarshaNo ratings yet
- Dissociation Constant of Citric Acid - 2Document21 pagesDissociation Constant of Citric Acid - 2Bhavesh JaniNo ratings yet
- Experiment - 1: Determination of Strength of An Acid Using A PH MeterDocument16 pagesExperiment - 1: Determination of Strength of An Acid Using A PH MeterAman Kumar0% (1)
- Lab 7 Post Lab (AutoRecovered)Document5 pagesLab 7 Post Lab (AutoRecovered)Maisy BrouilletteNo ratings yet
- Zeynep ÇortuDocument3 pagesZeynep Çortuilikler-04.minikameraNo ratings yet
- Standardization BuffersDocument4 pagesStandardization BuffersHoàng TuấnNo ratings yet
Raw data for Expt. 3
Raw data for Expt. 3
Uploaded by
tsun long TangCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Raw data for Expt. 3
Raw data for Expt. 3
Uploaded by
tsun long TangCopyright:
Available Formats
Experiment 3: Buffer solutions
Data Sheet
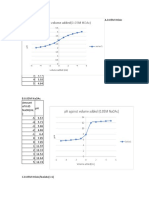
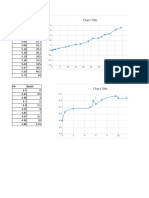
PART I. Experimental Results
Complete the following data table.
Mixture
A B C D E F G
Addition Volume
of Acid/Base of NaOH
pH
added,
Acid/ Volume (to two decimal places)
𝑽𝑵𝒂𝑶𝑯
Base
(mL) (mL)
none - 0.0 3.15 7.54 4.85 4.70 5.73 5.54 4.20
1.0* -1.0 2.81 6.28 4.78 4.61 2.90 5.43 4.05
2.0* -2.0 2.57 5.95 4.72 4.50 2.57 5.34 3.91
0.05 M
3.0* -3.0 2.43 5.73 4.65 4.22 2.41 5.25 3.77
HCl
4.0* -4.0 2.32 5.59 4.58 3.89 2.30 5.17 3.53
5.0* -5.0 2.23 5.48 4.52 3.35 2.21 5.10 3.20
- 0.0 3.10 7.52 4.85 4.81 8.57 5.57 4.18
1.0 1.0 3.44 11.46 4.90 5.05 11.43 5.70 4.23
0.05 M 2.0 2.0 3.75 11.79 4.96 5.32 11.79 5.90 4.33
NaOH 3.0 3.0 3.89 11.95 5.03 5.64 11.97 6.19 4.42
4.0 4.0 4.04 12.06 5.10 6.40 12.10 6.75 4.47
5.0 5.0 4.13 12.15 5.17 10.65 12.17 10.45 4.54
#Averag
none 0.0
e value
*For data analysis purpose, the volume of HCl added is viewed as the negative of the volume
of NaOH added.
# Average initial pH reading from the mixture before HCl or NaOH added.
A: 30 mL 0.05 M CH3COOH
B: 30 mL 0.05 M CH3COONa
C: 15 mL 0.05 M CH3COOH + 15 mL 0.05 M CH3COONa
D: 5 mL 0.05 M CH3COOH + 5 mL 0.05 M CH3COONa + 20 mL H2O
E: 30 mL H2O
F: 5 mL 0.05 M CH3COOH + 25 mL 0.05 M CH3COONa
G: 25 mL 0.05 M CH3COOH + 5 mL 0.05 M CH3COONa
You might also like
- PAG 11.2 - Acid Base Titration Curves GraphsDocument1 pagePAG 11.2 - Acid Base Titration Curves GraphsD ZooNo ratings yet
- Disassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide TitrationDocument19 pagesDisassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide Titrationwani280475% (4)
- Lab 15 - Acid-Base TitrationDocument5 pagesLab 15 - Acid-Base TitrationMelnykNo ratings yet
- Experiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Document15 pagesExperiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Noor Nasuha Noor Ariffin100% (1)
- CAPE - Chemistry LabsDocument19 pagesCAPE - Chemistry Labskelliann george100% (3)
- Graph of PH Against Volume of Naoh Titrated With Ch3Cooh: Equivalent Point: 29.20Document2 pagesGraph of PH Against Volume of Naoh Titrated With Ch3Cooh: Equivalent Point: 29.20adillaanisNo ratings yet
- PH and Buffer LabDocument13 pagesPH and Buffer LabAdellaine Lois GreyNo ratings yet
- CH Lab 3Document8 pagesCH Lab 3Mohammad Fahim NurNo ratings yet
- CH Lab 6Document7 pagesCH Lab 6Mohammad Fahim NurNo ratings yet
- Lab ReportDocument9 pagesLab ReportEssielve BatistilNo ratings yet
- Post-Lab Exer 1 PDFDocument5 pagesPost-Lab Exer 1 PDFDaniel Seth AndalNo ratings yet
- Lab ReportDocument6 pagesLab ReportShashaNo ratings yet
- Lab Report 1Document27 pagesLab Report 1szulkipeliNo ratings yet
- Lab 2 3Document11 pagesLab 2 3api-347340507No ratings yet
- Universiti Tunku Abdul Rahman Faculty of Science Bachelor of Science (Hons) ChemistryDocument6 pagesUniversiti Tunku Abdul Rahman Faculty of Science Bachelor of Science (Hons) ChemistryKirthinee JegatheesanNo ratings yet
- PH Against Volume Added (0.05M Hoac)Document4 pagesPH Against Volume Added (0.05M Hoac)Leroy ChengNo ratings yet
- CHM204 - Lab Report 2Document11 pagesCHM204 - Lab Report 2Romy MansourNo ratings yet
- Marcet Boiler Lab ReportDocument4 pagesMarcet Boiler Lab ReportJohnConor95% (41)
- Chem 2 Weak Base Strong Acid Lab ReportDocument6 pagesChem 2 Weak Base Strong Acid Lab ReportMohammad Izadi100% (1)
- Chem300Quiz 2ChEA LibutlibutDocument15 pagesChem300Quiz 2ChEA LibutlibutEredson LibutlibutNo ratings yet
- Exer 2 Post-Lab ReportDocument6 pagesExer 2 Post-Lab ReportKin DemoticaNo ratings yet
- Perhitungan Laboratorium-1Document11 pagesPerhitungan Laboratorium-1Akbar HidayatallahNo ratings yet
- Chemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaDocument18 pagesChemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaSita kumarNo ratings yet
- Lab 2 - (Titration)Document11 pagesLab 2 - (Titration)api-383698554No ratings yet
- Experiment 8Document5 pagesExperiment 88005Girish Kumar DasNo ratings yet
- Che485 - Exp 1Document21 pagesChe485 - Exp 1raisshakim02No ratings yet
- Neutralizacion NaOHDocument2 pagesNeutralizacion NaOHDarwin Laqui TiconaNo ratings yet
- AP CHEM Lab Acid Base Titration PDFDocument4 pagesAP CHEM Lab Acid Base Titration PDFMelnykNo ratings yet
- Preparation of PH Buffer SolutionsDocument5 pagesPreparation of PH Buffer SolutionscscsscNo ratings yet
- V Naoh (ML) PH: Otentiometric ItrationDocument9 pagesV Naoh (ML) PH: Otentiometric ItrationradyjrNo ratings yet
- CHEM Experiment 7Document11 pagesCHEM Experiment 7Mary Gencel EstrellaNo ratings yet
- Preparation of PH Buffer SolutionsDocument5 pagesPreparation of PH Buffer SolutionsAndrew Sumaya AldeNo ratings yet
- Buffer SolutionDocument8 pagesBuffer SolutionAbubakar UmarNo ratings yet
- Lab Report BoiDocument7 pagesLab Report BoiNORHIDAYATI BINTI MD GHAZALI MoeNo ratings yet
- Su AlbuDocument7 pagesSu AlbuLama SalahatNo ratings yet
- DkaDocument4 pagesDkaAlif Alfarisyi SyahNo ratings yet
- Tabla de Datos Procesados EVI Biología FinalDocument20 pagesTabla de Datos Procesados EVI Biología FinalYovanni SanchézNo ratings yet
- Chart TitleDocument4 pagesChart TitleBrigitte PorezNo ratings yet
- CHAPTER III AnachemDocument4 pagesCHAPTER III AnachemNur-Zhiana MuhiddiniNo ratings yet
- Chart Title: PH HCL PH Naoh 1 2 3 4 5 6 7 8 9 10 15 20 25 ML Adiciona DosDocument3 pagesChart Title: PH HCL PH Naoh 1 2 3 4 5 6 7 8 9 10 15 20 25 ML Adiciona DosSara Cicero RodriguezNo ratings yet
- Post Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Document7 pagesPost Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Mohammad Fahim NurNo ratings yet
- Phyical Lab CurvesDocument9 pagesPhyical Lab Curvescyrilbas16No ratings yet
- Estandarizacion NaohDocument10 pagesEstandarizacion NaohValeNo ratings yet
- Buffers: Lab ReportDocument8 pagesBuffers: Lab ReportjahangeerNo ratings yet
- CM 1502 Lab 3Document16 pagesCM 1502 Lab 3i000yearsNo ratings yet
- Estimation of Active IngredientDocument5 pagesEstimation of Active IngredientSuleman QaiserNo ratings yet
- Water Lab ReportDocument8 pagesWater Lab ReportMonica GellerNo ratings yet
- Datos Potenciometria 25-05-23Document6 pagesDatos Potenciometria 25-05-23Angela VillegasNo ratings yet
- Lab Report 3 KotDocument15 pagesLab Report 3 KotNikMuhammadIzzatNo ratings yet
- CAPE - Chemistry LabsDocument19 pagesCAPE - Chemistry Labskelliann georgeNo ratings yet
- Experiment 2Document6 pagesExperiment 2Sinichi IzumiNo ratings yet
- Determination-Of-Freezing-Point-DepressionDocument8 pagesDetermination-Of-Freezing-Point-DepressionAllan TampusNo ratings yet
- Chemical Reaction Engineering Laboratory: Title of Experiment: Production of CODocument10 pagesChemical Reaction Engineering Laboratory: Title of Experiment: Production of COHarshaNo ratings yet
- Dissociation Constant of Citric Acid - 2Document21 pagesDissociation Constant of Citric Acid - 2Bhavesh JaniNo ratings yet
- Experiment - 1: Determination of Strength of An Acid Using A PH MeterDocument16 pagesExperiment - 1: Determination of Strength of An Acid Using A PH MeterAman Kumar0% (1)
- Lab 7 Post Lab (AutoRecovered)Document5 pagesLab 7 Post Lab (AutoRecovered)Maisy BrouilletteNo ratings yet
- Zeynep ÇortuDocument3 pagesZeynep Çortuilikler-04.minikameraNo ratings yet
- Standardization BuffersDocument4 pagesStandardization BuffersHoàng TuấnNo ratings yet