Professional Documents
Culture Documents
C2018-10490
C2018-10490
Uploaded by
tinovelazquezCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C2018-10490
C2018-10490
Uploaded by
tinovelazquezCopyright:
Available Formats
Paper No.
10490
Elucidating the Corrosion Phenomena in a Thermal In Situ Oil Sands Emulsion Pipeline
Ayodele O. Okunola, Randy Damant & John A. Lerbscher
Baker Hughes, a GE Company
5050 47 Street SE
Calgary, AB T2B 3S1
Canada
ABSTRACT
For thermal in-situ oil sands production, it is conventional to think that an emulsion pipeline remains
essentially oil-wet. Ideally, an oil coating distributed on the pipe by the produced water-in-oil emulsion
from a well pad is expected to give the needed corrosion protection for the operating life of the pipeline.
Practically however, there are conditions under which this ideal scenario becomes no longer feasible,
even for low API gravity heavy oil. These parameters affecting protection include but are not limited to
water cut, well pad processing, steam cycle phenomena, reservoir characteristics, pipeline operating
temperature, partitioning characteristics of the acid gases and their effects on water chemistry and
passivation as well as other field operational practices.
From the experience of two case histories at a thermal in-situ oil sands project, this paper elaborates on
many of the field parameters and how they influence the integrity of pipeline infrastructure by studying
the various corrosion phenomena at play. Corrosion mitigation recommendations for these pipelines will
also be presented.
Key words: thermal in-situ, oil sands, asset integrity, corrosion.
INTRODUCTION
Consideration of corrosion mechanisms in thermal oil recovery facilities should be divided into two
categories, conditions with and without microorganisms present; the former is called microbiologically
influenced corrosion (MIC). Constant monitoring of these facilities for MIC and controlling them when
present is essential to corrosion management. If corrosive microorganisms are present in significant
amounts, the probability of failure is high despite the presence of other corrosion mitigating factors
including assumptions about oil-wetting, passive scale formation or the efficacy of a chemical inhibition
program. This paper deals with non-microbiological corrosion issues and considers the following factors
in the context of thermal oil production:
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
1
Evolving steam cycle phenomena that affect oil-wetting and the nature of produced emulsions
including specific gravity.
Flow regimes and oil/water ratios.
Water chemistry as effected by dissolved solids, acid gases, oxygen ingress and the presence of
low molecular weight organic acids.
Thermodynamic considerations based on pH and electrochemical oxidation-reduction potential
(ORP) as diagramed in Pourbaix format.
Effects of temperature and pressure changes.
The effect of accumulated solids.
Contamination and temperature lowering by injecting surface water and/or trucked in fluids into
the emulsion lines.
Effects of injecting oxygen laden vapor recovery unit (VRU) liquids into the emulsion lines.
Electronic Corrosion Engineer1 (ECE) is used to model expected corrosion rates under a selection of
conditions assuming water-wetted surfaces are present.
Thermal Oil Production
Cyclic Steam Stimulation (CSS) and Steam Assisted Gravity Drainage (SAGD) are two of the most widely
applied technologies for the in-situ extraction of heavy oils from an oil sands bitumen formation, with the
CSS technology predating the SAGD. Unlike the continuous process of the SAGD, CSS involves a three
stage process. The first stage is a period of high pressure and high temperature wet steam injection,
below the formation fracture pressure, lasting several weeks, depending on process design and reservoir
characteristics from where the bitumen is produced. The second stage is that of soaking, whereby the
injected steam is allowed to heat up the cold bitumen in the formation to achieve mobilization by reducing
viscosity. The third stage involves the production of bitumen, often supported by pumps. The injection
and production phases together comprise one cycle. Steam is re-injected to begin a new cycle when oil
production rates fall below a critical threshold due to the cooling of the reservoir. Another major difference
is that the SAGD production process involves a twin well system. High pressure dry steam is injected
through one well and the mobilized bitumen drains into the production well by gravity.
The CSS can also be performed at pressures above the formation fracture pressure in the so called High
Pressure Cyclic Steam Stimulation (HPCSS), now used by roughly 35% of all thermal in-situ production
in the Alberta oil sands. In this technique, steam is injected at a higher pressure to achieve a fracture in
the formation thereby further improving the flow characteristics of the bitumen.
The preferred in-situ extraction method is ultimately determined by economic and environmental factors,
many of which have been covered by several authors2. The prevailing low crude oil price environment
along with newer stringent environmental considerations are pushing the frontiers even further beyond
the well-known factors.
The corrosion phenomena in a CSS oil sands emulsion pipeline varies along the different stages of a
typical production cycle. This variation is dependent on the number of cycles that have gone in, how long
a well has produced and how much a reservoir has been depleted.
According to conventional thinking, it is understood that robust passive iron carbonate layers formed at
high temperatures will remain essentially oil-wet. This combination is expected to provide adequate
corrosion protection for the operating life of the pipeline. Due to a number of factors however, it cannot
be reliably assumed that no water wetting will occur and that the iron carbonate layer will remain robust.
Once a water-wet metal surface is established, a number of corrosion mechanisms become possible.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
2
Root cause analyses of failures in two emulsion pipelines lead to an overview of the corrosion potential
as conditions evolve and suggestions are made towards establishing best practices.
CORROSION FACTORS
Steam Cycle Phenomena
In cyclic steam stimulation, a steam cycle is defined as the time frame from the start of one steam injection
to the beginning of the next, within which a soaking period and some bitumen production has been
completed. The next cycle of steam injection is necessitated by a depletion in production due to the
increased immobility of the bitumen as a result of cooling of the formation. During a typical cycle, 10%
of the calendar days are for steam injection, 10% for soak, and 80% are for production operations 2. As
a consequence of steam injection, improved mobility of the heavy bitumen leads to flow in the near
wellbore relative to locations further into the formation. Three distinct and sequential flow regimes have
been known to characterize the production phase3. Type I involves free water with very little bitumen
production. In type II, slugs of water alternate with slugs of water-in-bitumen emulsion resulting in
scattered production rates. Type III is a single phase flow of water-in-bitumen emulsion with consistent,
but slowly varying production rates.
In early steam cycles, a significant portion of the produced bitumen occurs during type III flow, with type
II flow regime being very small. Here, emulsification of water into the bitumen swells the bitumen to
occupy the whole pore space, resulting in a single phase emulsion flow4. As the cycles increase, the
water content of the emulsion in type III flow reaches a maximum of about 50% and the flow is then
characterized by a type II regime. This also corresponds to a decline in the well’s oil to steam ratio (OSR)
and an increase in the water/oil ratio (WOR). In many practical cases, the bitumen produced from cyclic
steam stimulation is essentially a water in oil emulsion, with the water content ranging from about 15%
to 50% by volume. Depending on the prevailing flow regime, there is additional free water accompanying
the bitumen emulsion bringing the total water cut of the produced fluids to between 70 – 80%.
It is known that there is a significant gas saturation in oil sands reservoirs, leading to the foamy nature of
the produced emulsion, with methane being the main hydrocarbon gas produced5. The carbonate rich
nature of the Cold Lake and Peace River formations in western Canada, from where most commercial
thermal in situ bitumen extraction takes place, makes the produced methane rich in CO 2.
Low API gravity crude from Canadian oil sands has a very healthy emulsion breakpoint, which is an
expression of the ability of an oil to form emulsion with water. It is also defined as the amount of water
above which the emulsion will separate. Emulsions with water contents below this point should not water-
wet pipeline surfaces, reducing risk of corrosion during a type III flow regime where no free water is
expected. As more steam cycles are completed, especially for mature CSS wells, the possibility of type
II flow regimes increases significantly. Areas of water wet steel can be established which may be difficult
to make oil-wet again particularly those at 6 O’clock where free water accumulates.
Possible Passivation Conditions
Many good articles on CO2 corrosion have been published in recent years where new methods are used
to investigate compositions of corrosion by-products after exposure to specific environments. As well,
software is available to construct Pourbaix Diagrams where thermodynamic stabilities for specific
systems can be developed. These publications give us good insight into potential consequences of
changing conditions in thermal oil production. Three articles recently published by Nesic et al 6 - 8 are
good examples where temperature dependent corrosion studies are coupled with analyses of corrosion
by-products and Pourbaix Diagrams constructed for experimental conditions. The relevant diagrams are
reprinted with permission and shown in Figure 1. The black-filled circles in plates b through f represent
this system at pH 4 while the white-filled circles are at pH 6. Features to note are:
Thermodynamic regions where solid corrosion by-products are stable change significantly with
temperature.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
3
Small changes in pH can transition the system from states where corrosion by-products form
solids on the metal surface to states where no solids form.
SEM photographs (not reproduced here) clearly show how the physical nature of corrosion by-
products change with temperature and pH. Higher temperatures and higher pH’s foster stable,
robust corrosion by-product layers while lower temperatures and pH’s may put the system into an
area where no corrosion solids are expected to form and, if they do form, they may not be robust.
As the temperature increases, the solubility of FeCO 3 shifts to the left increasing the possibility of
precipitation at lower pH. This means FeCO3 is stable at lower pH provided the temperatures are
high enough.
Below 80 °C, the most stable form of iron in acidic conditions is Fe2+, but in neutral and alkaline
conditions, FeCO3 is the most stable form. As the temperature increases to 250 °C, Fe 3O4 will be
the most stable product in neutral and alkaline conditions.
Nesic’s experimental environments are chemically pure and relatively simple compared to those in real
producing systems but the features above still apply. Pipelines carrying very hot emulsion and free water
are likely to be operating in a region of thermodynamic stability where robust by-product films are formed.
As temperatures drop, CO2 becomes more soluble in all phases lowering the pH - perhaps only slightly
if a bicarbonate buffer is present. Not only are corrosion rates affected but the by-products may not form
at all or, if they do, they will not form robust, nicely packed crystalline layers. Producers should be aware
of these transitions and be prepared to escalate corrosion mitigating measures as their systems approach
these transitional conditions.
pH and Temperature Considerations
Unless perturbed by renegade acid flow back from stimulation procedures or by the addition of acidic
demulsifying chemicals, the pH is determined by temperature, pressure and the presence of acidic
species such as CO2, H2S and naturally occurring organic acids. The effects of pH can be understood
from two main perspectives: the effect it has on the rate of corrosion mechanisms and the effect it has
on iron carbonate passivation (kinetics and thermodynamics).
Acid Gases and Organic Acids
For thermal in-situ oil sands extraction, regular bitumen emulsion production is accompanied by methane
and CO2. For more mature assets, the produced gas become increasingly sour due to the production of
H2S as a result of thermochemical sulfate reduction, whereby the interaction of steam and sulfur
containing bitumen at high pressure and temperature leads to the liberation of H 2S. For a given aqueous
phase with a consistent level of dissolved solids, the pH becomes a function of dissolved acid species
and temperature. CO2 and H2S partition between the gas, oil and free water as per their thermodynamic
solubility characteristics and that solubility in the aqueous phase is a function of temperature, partial
pressures and salinity.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
4
Figure 1: As reprinted with permission
Molecular forms of low molecular weight organic acids such as formic, acetic and propionic partition
between the oil and water according to another set of thermodynamic relationships. The molecular forms
dissociate in water and lower the pH while anions of these acids (formate, acetate and propionate) only
reside in the aqueous phase and have been shown to enhance corrosion rates beyond certain threshold
concentrations9.
The partitioning characteristics of CO2 and H2S between the bitumen-entrained water, pure bitumen and
free water depend on temperature, pressure and total dissolved solids that will change between reservoir
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
5
and surface environments. The combined relationships between temperature, pressure, pH and acid
gases, particularly CO2, are reviewed below.
As a first look, the solubility’s of CO2, H2S and O2 are estimated using an in-house calculator10 and results
shown in Figures 2, 3 & 4. pH vs temperature graphs were then constructed at different mole percent
CO2 levels in a representative gas phase using a well-accepted calculator11 that uses thermodynamic
activities and equilibrium constants adjusted for temperature. The model brine composition and system
conditions used are shown in tables 1 & 2; gas specific gravity was adjusted as per the average mol. wt.
of the model gas. Results are shown in Figure 5. These graphs show how gas solubility increases and
pH drops as the temperature drops for any given set of conditions. At first glance, the pH changes seem
too small to matter but in the context of the Pourbaix diagrams shown in Figure 1, we see these changes
are occurring close to where boundaries of corrosion by-product stabilities are also changing with
temperature. The SEM surface imaging sections of Nesic’s papers7 show that by-product layer
morphologies change from robust to non-passivating as temperatures drop.
Figure 2: CO2 Solubility at 1000 kpa in 0.17 molal NaCl Equivalent Solution
Table 1: Model Brine Composition Used in the pH Calculations
Species Conc (mg/L)
Na+ 3,898
Cl- 6,800
Ca+ 328
HCO3-1 500
TDS 12,040
Table 2: System Conditions Used in the pH Calculations
Variable Value
Gas Production (mmSm3/day) 0.5
Oil Production (m3/day) 175
Water Production (m3/day) 408
Ptotal (bar/psi) 12 / 166
Percent H2S (mole %) 0.25
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
6
Figure 3: H2S Solubility at 0.25 mol% in 1000 kpa Total Pressure in 0.17 NaCl Molal Equivalent
Solution
Figure 4: Dissolved Oxygen Concentration in a 0.17 Molal Solution in Contact with Gas at
1000 kpa containing 200 ppm Oxygen.
Nesic, et al 6, 7 concluded the following based on the two cited research reports:
The higher the pH, the less corrosive the system is because there are fewer H + ions and the
solubility of FeCO3 decreases which fosters rapid corrosion by-product layer formation.
Lower pH environments lead to higher corrosion rates for the entire temperature range studied
(80-200oC)
At a fixed pH, corrosion rates decrease at temperatures above 120 °C due to the formation of
robust (passivating) layers of FeCO3.
At temperatures above 150 °C, small amounts of Fe3O4 were detected along with FeCO3 in the
passive film, both at pH 4.0 and 6.0.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
7
Figure 5: pH vs T Calculated Using Selected Levels of CO2 in the Associated Gas
The enhancing effects of low molecular weight organic acids (LMWOA’s) on CO 2 corrosion have been
well investigated.9, 12 – 18. Given the level of total LMWOA’s acids in this field, higher corrosion rates are
expected than those based on acid gas alone. ECE captures this effect and results are shown in Figure
7. A more specific analysis of LMWOA’s in systems like this exceeds the scope of this paper.
Effects of Introducing Non-produced Fluids
A typical pad configuration and failure locations are shown in Figure 6. Emulsion pipelines are commonly
used to dispose of surface waters accumulated within the berm structure from rain or melting snow. This
water must be kept within the confines of berms built to isolate well pads from unleased land and needs
to be removed from time to time. A cost effective and convenient approach is to accumulate it in open
tanks and inject it into the emulsion pipelines for transport to disposal wells via the main processing
facility. Also, condensed liquids from a vapor recovery unit are added to the emulsion line.
An open vent surface water accumulation tank is tied into the emulsion line, upstream of the vapor
recovery unit (VRU) inlet. This introduces very cold water into the emulsion line and may contain
trucked in fluids from other pads.
Vapor recovery units (VRU’s) condense escaping vapors and return additional cold liquids to the
emulsion line. The condensed liquids contain both water and light hydrocarbons and it should be
noted that oxygen is about ten times more soluble in light hydrocarbons than in water. Unless the
VRU is blanketed to prevent air ingress, these liquids will contain considerable amounts of
dissolved oxygen (DO) especially in colder seasons.
All these fluids are cold and will have high levels of dissolved oxygen creating two consequences
when injected into the hot emulsion.
o In general, they lower the temperature of the emulsion that persists for the duration of the
flow; effects of lower temperature have been discussed above.
o The cold, oxygen-containing fluids will be instantly heated and release their dissolved
oxygen which will partition into the emulsion, the free water and the associated gas. Based
on experience, high corrosion rates are typical immediately downstream of this
phenomenon.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
8
Figure 6: Typical pad configuration and failure locations with dates
Introducing low levels of DO into a system already corroding due to acid gases is observed to be
unusually pernicious immediately downstream. This is attributed to a cathodic depolarization effect
where the oxygen reacts rapidly with adsorbed and absorbed atomic and molecular hydrogen species
accumulated on cathodic areas thereby facilitating the reduction of H + ions which increases the cathodic
current. When these cathodic areas are connected to anodic areas under a deposit or in an existing pit,
localized metal dissolution rates increase in those pits which, in turn, enhances associated acidified
pitting mechanisms19. In cases of chronic ingress, oxygen has additional effects.
The oxygen provides an additional cathodic reaction.
The solution ORP may increase to the point where Fe3+ ions are created in significant amounts.
When exposed to the bulk solution, these ions form solids such as FeO(OH) that now interfere
with solids formed by Fe2+ such as FeCO3 and Fe3O4. When created inside of a pit, the
acid-creating hydrolysis of Fe3+ is much stronger than that from Fe2+ ions which, in turn,
accentuates acidified pitting mechanisms.
Lower fluid temperatures in the emulsion lines allow the growth of microorganisms. Unless treated with
biocide, microorganism contamination will result and once colonies are formed, localized corrosion is
likely. Anodic areas are established underneath, and cathodic areas at the periphery. Acid producing
bacteria (APB), identified in one of the failures, are able to lower the pH under the biomass which
destabilizes any protective FeCO3 scale formed. Additionally, cathodic areas around the anodic sites
created by microorganism colonies will be depolarized by oxygen, thereby enhancing pitting corrosion
rates.
Summary
Considering the evolving conditions in thermal oil fields, we can now see the bigger picture.
Initially, hot (T > 125 °C) type III production dominates. 100% of the pipe is expected to be
oil-wetted, any corrosion layers are expected to be robust, pH’s are expected to be higher for a
given set of conditions and corrosion rates for any water-wetted areas (if present) are expected
to be low.
o Flow volumes of water-in-oil emulsion are high relative to pipe size; high flow rates
combined with the significant viscosity of this emulsion work to sweep the lines of
accumulated debris.
o No common oil field microorganism can thrive at these temperatures and the solubility of
oxygen, if present, will be minimal
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
9
As production progresses to type II, the probability of water-wetted areas increases but high
temperatures may persist mitigating potential corrosion mechanisms.
o Once a water-wetted area is established on the pipe bottom, the probability of oil-rewetting
is low in the absence of a corrosion inhibitor.
o Even though total flow volumes may remain high, increasing amounts of free water occupy
the lower pipe quadrant; water is much less viscous and will not sweep debris as efficiently
as emulsion. Solids that begin to accumulate will foster initiation of under-deposit acidified
pitting mechanisms that will proceed slowly under these conditions. Routine pigging and
the use of corrosion inhibitors eliminates this problem.
o Temperatures are generally high enough to prevent microorganism growth.
Water-wetting is highly probable in type I production.
o Total flow volumes are lower, further increasing the probability of solids accumulation on
pipe bottoms.
o Temperatures are most likely to fall during this period
As temperatures fall, regardless of the flow type, key parameters adjust as discussed.
o Acid gases become more soluble and pH’s go lower
o Oxygen becomes more soluble and any ingress becomes pernicious because polarized
cathodes surrounding young, under-deposit pitting situations are depolarized by oxygen
enabling corrosion current to flow between anodic and cathodic areas. This enhances
acidified pit mechanisms boosting pitting corrosion rates to dangerous levels.
o The morphology of corrosion by-products becomes less robust.
o Parts of the system may cross a thermodynamic boundary where no solid corrosion
by-products are formed.
The combined effect of these adjustments will transition parts of the system to a potentially more
corrosive state as well as enabling the onset of MIC if the pipe becomes contaminated.
CORROSION MODELLING
Electronic Corrosion Engineer version 5.2.2 software1 was used to establish general and pitting corrosion
rate reference points to compare with empirical information gathered. Inputs to this software were
extracted from the Pipeline Corrosion Risk Ranking document and other available information.
In addition to the normal operating conditions and throughput data, temperature variations were modelled
to show the effects of temperature on calculated pH as discussed above. Calculated pH values represent
the pH at metal-liquid interfaces where corrosion occurs and are in line with those calculated earlier using
ScaleSoftPitzer. The model incorporates dissolved salts of carbonates and bicarbonates into its pH
calculations as well as the overall ionic strength of the aqueous phase. The model assumes:
• A closed system completely free of oxygen either from the VRU or from the vent tank.
• There are also no trucked-in fluids from surface waters introducing MIC.
• A uniform temperature at any given point in the pipeline section being modeled.
The role of organic acids in the CO2 corrosion mechanism was also examined using produced water
analysis data. To highlight the effects, isolated pitting corrosion rates in the presence and absence of
organic acids for one of the pipeline segments were compared for illustrative purposes in Figure 7.
Schematics of the emulsion gathering system and piping specifics are given in Figures 8 & 9. One set of
operating conditions was used for all pipelines and is summarized in Table 3.
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
10
Figure 7: Isolated Pitting Corrosion Rates as a Function of Temperature for Line Segment 1 in
With and Without Organic Acids
Figure 8: Pipeline Schematic
Figure 9: Thermal Expansion Features
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
11
Table 3: Field operating conditions applied for corrosion modelling
Parameter Value
Temperature 10 - 150 °C
Pressure 158 – 174 psi
Oil Flow Rate 24 - 252 Sm³/d
Gas Flow Rate 0.320 - 1 MMSm³/d
CO2 in gas phase 42 mol%
H2S in gas phase 0.25 mol%
Chlorides 6800 ppm
Bicarbonates
500 ppm
(dissolved)
Water Cut 60 – 89%
Crude Oil Flow Rate 204 Sm³/day
API Gravity 21 °API (0.927 g/cm³)
Total Organic Acids 442 mg/L
Gas Flow Rate 0.32 MMSm³/day
In line segment 1 (OD: 219.1 mm, WT: 3.2 mm, L: 270 m), starting from a scenario of 10 °C, the pitting
corrosion rate is predicted to be about 262 mpy, which then increases steadily until 70 °C (Figure 10).
This was observed to be a critical temperature, beyond which the corrosion rates begin to drop. This
phenomenon was discussed above and has been observed in many laboratory and field cases 20. The
corrosion rates continued to drop as temperatures were further increased. pH values calculated by this
model are in line with those calculated earlier using ScaleSoftPitzer SSP 2017. The same was repeated
for segments 2 (3.81 km), 3 (490 m) and 4(1.05 km) which all have an outer diameter of 406.4 mm and
a wall thickness of 6.4 mm. Results are presented in Figures 11 to 13.
Figure 10: A plot of isolated pitting corrosion rates and pH as a function of temperature in line
segment 1 (see also Figure 8).
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
12
Figure 11: A plot of isolated pitting corrosion rates and pH as a function of temperature in
line segment 2 (see also Figure 8).
Figure 12: A plot of isolated pitting corrosion rates and pH as a function of temperature in line
segment 3 (see also Figure 8).
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
13
Figure 13: A plot of isolated pitting corrosion rates and pH as a function of temperature in line
segment 4 (see also Figure 8).
CONCLUSIONS
The evolution of flow regimes inevitably leads to water-wetted surfaces.
The modeled results are in line with the above discussion on how temperature affects gas
solubility’s, pH, passive layer morphology and the thermodynamic stability boundaries of various
passivating solids in the absence of oxygen ingress or microorganism activity.
Significant corrosion failures can be expected as temperatures fall.
Continuous excursions from passivating conditions, oxygen ingress or microorganism activity
make the need for chemical treatments and pigging inevitable.
Recommendations
1. Emulsion lines should be routinely pigged when free water cuts exceed 20% and/or when
temperatures fall below 100 °C.
2. Oxygen tolerant chemical inhibitors should be considered when temperatures fall below 100 °C.
3. VRU’s should be blanketed to eliminate that function as a source of oxygen ingress.
4. Surface water should be filtered before being stored in accumulator tanks.
5. Tanks accumulating surface water should be treated with biocide and microorganism counts
monitored at all key locations.
6. Preheating the stored surface water with steam before injection will serve two functions.
a. Much of the dissolved oxygen will be removed.
b. The emulsion lines will remain consistently hotter.
REFERENCES
1. L. Smith and K. DeWaard, “Corrosion Prediction and Material Selection for Oil and Gas Producing
Environments”, CORROSION/2005, paper no. 05648 (Houston, TX: NACE, 2005).
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
14
2. G. R. Scott, “Comparison of CSS and SAGD Performance in Clearwater Formation at Cold Lake”,
SPE/PS-CIM/CHOA, paper no. 79020, (Calgary, AB: SPE, 2002).
3. E. Vittoratos, “Flow Regimes During Cyclic Steam Stimulation at Cold Lake”, The Journal of Canadian
Petroleum Technology, vol. 30, no. 1, 1991, pp. 82 – 86.
4. E. Vittoratos, “Interpretation of Production Data from Cyclic Steam Stimulation at Cold Lake”, 65th
Annual Technical Conference and Exhibition, paper no. 20527 (New Orleans, LA: SPE, 1990), pp.
569 – 574.
5. B. T. Reilly and G. R. Scott, “Cold Lake Project Recovery and Role of Foamy Emulsion”, International
Heavy Oil Symposium, paper no. 30287 (Calgary, AB: SPE, 1995), pp. 403 – 411.
6. T. Tanupabrungsun, B. Brown and S. Nesic, “Effect of pH on CO2 Corrosion of Mild Steel at Elevated
Temperatures”, CORROSION/2013, paper no. 2348, (Orlando, FL: NACE, 2013).
7. T. Tanupabrungsun, D. Young, B. Brown and S. Nesic, “Construction and Verification of Pourbaix
Diagrams for CO2 Corrosion of Mild Steel Valid up to 250 °C”, CORROSION/2012, paper no. C2012-
0001418 (Salt Lake City, UT: NACE, 2012).
8. S. Nesic, “Key Issues Related To Modelling of Internal Corrosion of Oil and Gas Pipelines – A
Review”, Corrosion Science, vol. 49, no. 12 (2007), pp. 4308 – 4338.
9. J.L. Crolet, N. Thevenot and A. Dugstad, “Role of Free Acetic Acid on the CO 2 Corrosion of Steels”,
CORROSION/99, paper no. 24, (Houston, TX: NACE, 1999).
10. Polynomial regression was applied to a variety of published gas solubility data sets to create a
calculator that estimates concentrations of CO2, H2S and O2 in aqueous solutions with salinities
between 0 and 45. Aqueous phases in thermal oil may be different from these idealized cases but
the trends represent the solubility characteristics under discussion.
11. ScaleSoftPitzer SSP2017
12. J.L. Crolet and M.R. Bonis, “Why so low free Acetic Acid Thresholds in Sweet Corrosion at Low
PCO2?”, CORROSION/2005, paper no. 05272, (Houston, TX: NACE, 2005).
13. V. Fajardo, C. Canto, B. Brown and S. Nesic, “Effect of Organic Acids in CO 2 Corrosion”,
CORROSION/2007, paper no. 07319, (Houston, TX: NACE, 2007).
14. Y. Garsany, D. Pletcher, D. Sidorin and W.M. Hedges, “Quantifying the Acetate-Enhanced Corrosion
of Carbon Steel in Oilfield Brines”, Corrosion Science, vol. 60, no. 12 (2004), pp. 1155 – 1166.
15. R.L. Martin and P.D. Logan, “Laboratory and Field Study of the Influence of Simple Carboxylates on
Oilfield Corrosion and Inhibition”, International Symposium on Oilfield Corrosion, paper no. 95092
(Aberdeen, U.K. SPE, 2005).
16. S.S. Bhat, A. Isor, M. Kakoti, A.G. Sarkar and G.R. Sanjiva, “Volatile Fatty Acid-Enhanced Carbon
Dioxide Corrosion of a Multiphase Well Fluid Pipeline”, Materials Performance (January, 2005): pp.
52 – 55.
17. M.C. Ismail and S. Turgoose, “Prediction Equation of CO2 Corrosion With the Presence of Acetic
Acid”, International Oilfield Scale Symposium, paper no. 100412 (Aberdeen, U.K. SPE, 2006).
18. W. Dumie and D. Harrop, “Our Water Chemistry Contains Organic Acids – So What Does it mean?”,
International Oilfield Corrosion Symposium, paper no. 100669 (Aberdeen, U.K. SPE, 2006).
19. J.R. Galvele, “Mechanisms of Passivity Breakdown of Pure Iron as Compared to Other Metals in
Passivity and its Breakdown on Iron and Iron Base Alloys”, eds. R.W. Staeche, H. Okada
CORROSION/1976, (Houston, TX: NACE, 1976), pp. 118 – 120.
20. R.C. Woollam and S.E. Hernandez, “Assessment and Comparison of CO 2 Corrosion Prediction
Models”, International Oilfield Corrosion Symposium, paper no. 100673, (Aberdeen, Scotland, UK,
SPE, 2006).
©2018 by NACE International.
Requests for permission to publish this manuscript in any form, in part or in whole, must be in writing to
NACE International, Publications Division, 15835 Park Ten Place, Houston, Texas 77084.
The material presented and the views expressed in this paper are solely those of the author(s) and are not necessarily endorsed by the Association.
15
You might also like
- Understanding Advanced Chemistry Through Problem Solving The Learners Approach (Volume 1) - Revised Edition (Kim Seng Chan, Jeanne Tan) (Z-Library)Document339 pagesUnderstanding Advanced Chemistry Through Problem Solving The Learners Approach (Volume 1) - Revised Edition (Kim Seng Chan, Jeanne Tan) (Z-Library)loxadegoNo ratings yet
- FCCU Reactor and Transfer Line CokingDocument4 pagesFCCU Reactor and Transfer Line Cokingsaleh4060No ratings yet
- 01.prevention and Safe Clearing of Ash Build-Up in Boiler Bottom Ash HoppersDocument13 pages01.prevention and Safe Clearing of Ash Build-Up in Boiler Bottom Ash Hopperssekhar_ntpc100% (1)
- Reduce FoulingDocument6 pagesReduce FoulingJeEJyZaNo ratings yet
- Surface Inorganic Scale Formation in Oil and Gas IDocument12 pagesSurface Inorganic Scale Formation in Oil and Gas IROZANANo ratings yet
- OTC-24955-MS Maintaining Wellbore Integrity in Steam Injection Wells Using Fit For Purpose Cement Systems in OmanDocument9 pagesOTC-24955-MS Maintaining Wellbore Integrity in Steam Injection Wells Using Fit For Purpose Cement Systems in Omanatilio martinezNo ratings yet
- Casing LeaksDocument8 pagesCasing LeaksJames "Chip" NorthrupNo ratings yet
- MSDocument6 pagesMSAbed Alftah AgabNo ratings yet
- SPE 163085 Successfully Controlling Unwanted Gas Production in A Highly Naturally Fractured Carbonate ReservoirDocument5 pagesSPE 163085 Successfully Controlling Unwanted Gas Production in A Highly Naturally Fractured Carbonate ReservoirLeopold Roj DomNo ratings yet
- MECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashDocument8 pagesMECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashMikeNo ratings yet
- MECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashDocument8 pagesMECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashMikeNo ratings yet
- AIChE (Design Guidelines For Subsea Oil Systems)Document17 pagesAIChE (Design Guidelines For Subsea Oil Systems)platipus_85100% (1)
- Firmansyah Et Al. - 2016 - Transient Cooling Simulation of Atmospheric Residue During Pipeline ShutdownsDocument11 pagesFirmansyah Et Al. - 2016 - Transient Cooling Simulation of Atmospheric Residue During Pipeline ShutdownsAbraham SilesNo ratings yet
- Delayed Coker Fired Heater Designand Operation Fouling PDFDocument10 pagesDelayed Coker Fired Heater Designand Operation Fouling PDFMajid MohebbifarNo ratings yet
- Articulo 6Document12 pagesArticulo 6Juan carlos Martinez CarrilloNo ratings yet
- SPE 66536 Contaminated Water Production in Old Oil Fields With Downhole Water Separation: Effects of Capillary Pressures and Relative Permeability HysteresisDocument12 pagesSPE 66536 Contaminated Water Production in Old Oil Fields With Downhole Water Separation: Effects of Capillary Pressures and Relative Permeability HysteresisRONALDO JEREMY100% (1)
- Wax DepositionDocument35 pagesWax DepositionArjit KumarNo ratings yet
- Experimental - Investigation - of - Crude Oil DesaltingDocument19 pagesExperimental - Investigation - of - Crude Oil DesaltingElbahi DjaalabNo ratings yet
- SPE-182190-MS Optimization of Steamflooding Heavy Oil Reservoirs Under UncertaintyDocument24 pagesSPE-182190-MS Optimization of Steamflooding Heavy Oil Reservoirs Under Uncertaintyoppai.gaijinNo ratings yet
- SPE 166261 Distributed-Flux Burners Improve Life of Firetubes and Process Throughput in Heater TreatersDocument11 pagesSPE 166261 Distributed-Flux Burners Improve Life of Firetubes and Process Throughput in Heater TreatersDavid HerreraNo ratings yet
- Spe 71460 MS PDFDocument11 pagesSpe 71460 MS PDFLeonela PantojaNo ratings yet
- SPE 121143 Successful Field Application of A High-Temperature Conformance Polymer in MexicoDocument13 pagesSPE 121143 Successful Field Application of A High-Temperature Conformance Polymer in MexicoLeopold Roj DomNo ratings yet
- Penso 2017Document7 pagesPenso 2017Xavier BloombergNo ratings yet
- Polymer Bath Maintanence1 (1007)Document7 pagesPolymer Bath Maintanence1 (1007)Deepa MadavanNo ratings yet
- Spe 153967Document16 pagesSpe 153967Hans Forenza ArataNo ratings yet
- Boilertubefailures Cases JFAP 2015Document8 pagesBoilertubefailures Cases JFAP 2015jssukhadwalaNo ratings yet
- Scientific & Technical ReportDocument15 pagesScientific & Technical ReportMariam AsgharNo ratings yet
- Mitigation and Remediation Technologies of Waxy Crude OilsDocument35 pagesMitigation and Remediation Technologies of Waxy Crude OilsChandran UdumbasseriNo ratings yet
- Paper46 Hazards 23Document9 pagesPaper46 Hazards 23Nadia AfifahNo ratings yet
- Journal 39Document16 pagesJournal 39Rohammed CastilloNo ratings yet
- COND Cleaning and Leakage Inspection PDFDocument11 pagesCOND Cleaning and Leakage Inspection PDFprakashNo ratings yet
- Corrosion Prevention of Crude and Vacuum Distillation Column Overheads in Petroleum Refinery A Field Monitoring StudyDocument10 pagesCorrosion Prevention of Crude and Vacuum Distillation Column Overheads in Petroleum Refinery A Field Monitoring StudyNoorain AhmadNo ratings yet
- Current Overview of Cyclic Steam Injection Process: Johannes Alvarez, Sungyun HanDocument12 pagesCurrent Overview of Cyclic Steam Injection Process: Johannes Alvarez, Sungyun HanozmdzNo ratings yet
- Crude Oil Desalting ProcessDocument18 pagesCrude Oil Desalting Processiancu_jianu_60% (1)
- OBM - ElastomersDocument8 pagesOBM - ElastomersMarutpal MukherjeeNo ratings yet
- Acid Soluble Magnesia Cement: New Applications in Completion and Workover OperationsDocument7 pagesAcid Soluble Magnesia Cement: New Applications in Completion and Workover OperationsCoolProphetNo ratings yet
- Experimental Evaluation of Foams Stabilized by Ionic L - 2023 - Journal of CO2 UDocument9 pagesExperimental Evaluation of Foams Stabilized by Ionic L - 2023 - Journal of CO2 Uสราญศิริ วงศ์ศิริNo ratings yet
- SPE 134169 Designing and Testing Cement System For SAGD ApplicationDocument12 pagesSPE 134169 Designing and Testing Cement System For SAGD Applicationadvantage025No ratings yet
- Paraffin DepositionDocument10 pagesParaffin Depositionleomruiz100% (1)
- SPE 90688 Dilution Strategies For Wax Management and Control For Deepwater Development From A Flow Assurance Perspective: Part - Current Practice and PerspectiveDocument13 pagesSPE 90688 Dilution Strategies For Wax Management and Control For Deepwater Development From A Flow Assurance Perspective: Part - Current Practice and PerspectiveBlackCounterNo ratings yet
- ++a Review of Wax Mitigation Methods Through Hydrocarbon ProductionDocument11 pages++a Review of Wax Mitigation Methods Through Hydrocarbon ProductionSahin İmanov100% (1)
- Active Heated Pipe Technologies For Field Development Optimisation (OTC 26578-MS)Document21 pagesActive Heated Pipe Technologies For Field Development Optimisation (OTC 26578-MS)ArsénioNo ratings yet
- A Laboratory Study of Hot Carbon Dioxide Injection Into Fractured and Conventional CoresDocument13 pagesA Laboratory Study of Hot Carbon Dioxide Injection Into Fractured and Conventional Coresmsmsoft90No ratings yet
- 1.1 OverviewDocument5 pages1.1 OverviewOdofin GbengaNo ratings yet
- 2 BrasilDocument13 pages2 BrasilmaxjuliNo ratings yet
- SPE-187349-MS High Pressure Tertiary-CO2 Flooding in A Fractured Chalk ReservoirDocument27 pagesSPE-187349-MS High Pressure Tertiary-CO2 Flooding in A Fractured Chalk ReservoirAllanNo ratings yet
- The Effect of Corrosion On Crude Oil Distillation Plants 1715509028Document9 pagesThe Effect of Corrosion On Crude Oil Distillation Plants 1715509028Ahmed ELmlahyNo ratings yet
- Effect of Aging in Bituminous MixesDocument15 pagesEffect of Aging in Bituminous MixesyadavameNo ratings yet
- Yan 2010Document4 pagesYan 2010Karen MaryNo ratings yet
- An In-Depth Look at Polymer QuenchantsDocument4 pagesAn In-Depth Look at Polymer QuenchantsJader PitangueiraNo ratings yet
- IPTC 10562 Low-Dosage Hydrate Inhibitors (LDHI) : Advances in Flow Assurance Technology For Offshore Gas Production SystemsDocument8 pagesIPTC 10562 Low-Dosage Hydrate Inhibitors (LDHI) : Advances in Flow Assurance Technology For Offshore Gas Production SystemsWaleed Barakat MariaNo ratings yet
- SPE 165518 MS PIntegralCompletionTechniqueforHeavyOilThermalRecoveryDocument12 pagesSPE 165518 MS PIntegralCompletionTechniqueforHeavyOilThermalRecoveryjalestNo ratings yet
- spe-211560-msDocument17 pagesspe-211560-msMostafa MAHMOUD KORTAMNo ratings yet
- Need of Flow Assurance For Crude Oil PropertiesDocument8 pagesNeed of Flow Assurance For Crude Oil Propertiesvande852001No ratings yet
- China University of PetroleumDocument13 pagesChina University of PetroleumMIKIAS麦克No ratings yet
- HO FA Articulo5Document18 pagesHO FA Articulo5Porfirio AguileraNo ratings yet
- Corrosion MitigationDocument6 pagesCorrosion MitigationgshdavidNo ratings yet
- SPECIAL REPORT Molecular SieveDocument8 pagesSPECIAL REPORT Molecular Sievenitoxxx666No ratings yet
- A Perspective View of Flow Assurance in Deepwater Fields in BrazilDocument8 pagesA Perspective View of Flow Assurance in Deepwater Fields in BrazilmahmoodnazeriNo ratings yet
- Spe 152297 MSDocument6 pagesSpe 152297 MSLeopold Roj DomNo ratings yet
- Hydrostatic and Hydro-Testing in the Oil and Gas FieldFrom EverandHydrostatic and Hydro-Testing in the Oil and Gas FieldRating: 3 out of 5 stars3/5 (2)
- MithridatismDocument3 pagesMithridatismTodd WoosterNo ratings yet
- Development of Aluminium Scrap Melting Technology: © V. I. Gel, D. N. Rudakov UDC 669.054.8:669.71Document5 pagesDevelopment of Aluminium Scrap Melting Technology: © V. I. Gel, D. N. Rudakov UDC 669.054.8:669.71PrasanthanNo ratings yet
- Unit 5 NotesDocument25 pagesUnit 5 NotesShubham DubeyNo ratings yet
- Carbon Nano Particles. ReviewDocument16 pagesCarbon Nano Particles. Reviewankita awasthiNo ratings yet
- D Block ElementsDocument16 pagesD Block ElementsSreejay BommineniNo ratings yet
- Solcourse - Polythene DPM DatasheetDocument2 pagesSolcourse - Polythene DPM DatasheetkibzeamNo ratings yet
- Module 8 Stereochemistry Lecture 20 Stereochemistry I: NPTEL - Biotechnology - Cell BiologyDocument48 pagesModule 8 Stereochemistry Lecture 20 Stereochemistry I: NPTEL - Biotechnology - Cell Biologyr karthickNo ratings yet
- AP Chemistry - Classification of Matter Worksheet: Fill in The Blanks in The Table With The Words in The List BelowDocument3 pagesAP Chemistry - Classification of Matter Worksheet: Fill in The Blanks in The Table With The Words in The List Belowalma silvaNo ratings yet
- The Hand Forged Knife - Karl Schroen - 1984 (S)Document75 pagesThe Hand Forged Knife - Karl Schroen - 1984 (S)Alessandro Urenda100% (6)
- Paal-Knorr Pyrrole Synthesis: A. General Description of The ReactionDocument4 pagesPaal-Knorr Pyrrole Synthesis: A. General Description of The Reactionjorge esteban guerrero poloNo ratings yet
- BSC (Chem) Final Version Jan05Document50 pagesBSC (Chem) Final Version Jan05Matobola Joel MihaleNo ratings yet
- Las Shs Gen - Chem Melc 7 q2 Week-4Document12 pagesLas Shs Gen - Chem Melc 7 q2 Week-4Kim Francis Beluso Dollete IINo ratings yet
- 48.purolite A430MR CholestyramineResin ProductInformationDocument6 pages48.purolite A430MR CholestyramineResin ProductInformationAhmed ZidanNo ratings yet
- Chapter 1 Sem 1920Document60 pagesChapter 1 Sem 1920PMNo ratings yet
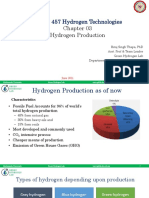
- MEPP 457 Hydrogen TechnologiesDocument88 pagesMEPP 457 Hydrogen TechnologiesAshim LamichhaneNo ratings yet
- Siri TriboelektrikDocument5 pagesSiri TriboelektrikRasailul AshwaqNo ratings yet
- Ions & Ionic Bonds (Multiple Choice) QPDocument7 pagesIons & Ionic Bonds (Multiple Choice) QPИРадојичић100% (1)
- Chapter3-pKa Table PDFDocument3 pagesChapter3-pKa Table PDFAnilesh BansalNo ratings yet
- Foggler - Wire Gauze ReactorDocument7 pagesFoggler - Wire Gauze ReactorJoshita KusumadewiNo ratings yet
- NCERT Practice SheetDocument3 pagesNCERT Practice Sheetbajrangkashyap2020No ratings yet
- Axel Quartzite 403Document2 pagesAxel Quartzite 403nsh.gnkinfraNo ratings yet
- Recent Trends in Chemistry of Pyridine N-OxideDocument10 pagesRecent Trends in Chemistry of Pyridine N-OxideNaresh KumarNo ratings yet
- One Pot Synthesis, Characterization of ZN GO Nanocomposite For The Electrochemical Decoloration of A Textile DyeDocument7 pagesOne Pot Synthesis, Characterization of ZN GO Nanocomposite For The Electrochemical Decoloration of A Textile DyeEditor IJTSRDNo ratings yet
- Tooth Paste Analysis CBSE Class 12Document23 pagesTooth Paste Analysis CBSE Class 12Radhey Patel100% (1)
- GUID - 6 en-USDocument37 pagesGUID - 6 en-USSantiago Cristancho100% (1)
- Unit 12MCQDocument7 pagesUnit 12MCQSwapnil SahuNo ratings yet
- g11 Module 1 in General Chemistry 1pdfDocument39 pagesg11 Module 1 in General Chemistry 1pdfAlthea EdulanNo ratings yet
- Unit One Material and Geometry of Cutting Tools 2015Document46 pagesUnit One Material and Geometry of Cutting Tools 2015elnat feyisa100% (1)
- Determination of CalciumDocument2 pagesDetermination of Calciumcocomelon8454No ratings yet