Professional Documents
Culture Documents
Manufacturer Page Rewritten - HTM
Manufacturer Page Rewritten - HTM
Uploaded by
api-894731Copyright:
Available Formats
You might also like
- Example of PM Interview PresentationDocument12 pagesExample of PM Interview PresentationAlex Gomez0% (1)
- SILC Working DraftDocument21 pagesSILC Working DraftBar & BenchNo ratings yet
- Stress Analysis Convergence Tips For - Dummies - CAE AssociatesDocument4 pagesStress Analysis Convergence Tips For - Dummies - CAE Associatessukhabhukha987No ratings yet
- Swim Ultra Efficient Freestyle WorkbookDocument140 pagesSwim Ultra Efficient Freestyle WorkbookMichel D100% (12)
- LAB 5 - Pineapple CordialsDocument17 pagesLAB 5 - Pineapple Cordialsghostly_form416995% (21)
- Giving Blood Scenario v2Document3 pagesGiving Blood Scenario v2api-894731No ratings yet
- Biologics Topic Page OnlyDocument5 pagesBiologics Topic Page Onlyapi-894731No ratings yet
- k162919 Device DescriptionDocument7 pagesk162919 Device DescriptionjsdanielinNo ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Digital Health Marketplace Summary For Canada 1635193267Document28 pagesDigital Health Marketplace Summary For Canada 1635193267arushi166No ratings yet
- Register Blood Bank Scenario v2Document4 pagesRegister Blood Bank Scenario v2api-894731No ratings yet
- FDA Easy on-PCDocument5 pagesFDA Easy on-PCVictor CuellarNo ratings yet
- FDA SofwaveDocument9 pagesFDA SofwavePhúc LâmNo ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Latin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)Document4 pagesLatin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)ComplianceOnlineNo ratings yet
- 2.2 (Rie Matsui - Eng) Vietnam WS (19oct2023)Document25 pages2.2 (Rie Matsui - Eng) Vietnam WS (19oct2023)tovanbac96No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Fda K161533Document11 pagesFda K161533Оксана КожокаруNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993ryan resultsNo ratings yet
- Small Business Qualification Certification GuidanceDocument31 pagesSmall Business Qualification Certification GuidanceNataliaNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document9 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Manoj NarukaNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993liuyonglogNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document11 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Gerardo DiazNo ratings yet
- USFundamentals Chapter36Document21 pagesUSFundamentals Chapter36Phan Do Dang KhoaNo ratings yet
- LaseMD LEO Laser System FDADocument9 pagesLaseMD LEO Laser System FDAakamteb.rdNo ratings yet
- K232431 - Brain Hemmorage - SiemensDocument8 pagesK232431 - Brain Hemmorage - Siemensmario.wisconsinNo ratings yet
- 510KDocument11 pages510Ksandy zhengNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document6 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993venkat_bhagavatiNo ratings yet
- 2019 IPI Winter Web CompressedDocument92 pages2019 IPI Winter Web CompressedKamran AlamNo ratings yet
- U .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Document5 pagesU .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Larissa GolucciNo ratings yet
- Solving Pharmas Quality Unit Identity CrisisDocument3 pagesSolving Pharmas Quality Unit Identity CrisisAYMEN GOODKidNo ratings yet
- Arkray Adams A1c Lite HA-8380V - FDA Datasheets and InterferenceDocument28 pagesArkray Adams A1c Lite HA-8380V - FDA Datasheets and Interferencemidifast2aNo ratings yet
- K 160412Document22 pagesK 160412Louelle HopeNo ratings yet
- Dia #1Document12 pagesDia #1Gaxi BofNo ratings yet
- 510 (K) Premarket NotificationDocument1 page510 (K) Premarket NotificationkuttyjNo ratings yet
- Register Blood Bank Scenario v4 Blood Topic PageDocument5 pagesRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731No ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document9 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Hau TyanNo ratings yet
- Helping Pharmas Manage Compliance Risks For Speaker ProgramsDocument9 pagesHelping Pharmas Manage Compliance Risks For Speaker ProgramsCognizantNo ratings yet
- Argos 510KDocument10 pagesArgos 510KNorman GuntsonNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993QFCarlosCQNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document13 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993ryan resultsNo ratings yet
- PD-400424 Rev B HolterCare 11.0.1 Release NotesDocument8 pagesPD-400424 Rev B HolterCare 11.0.1 Release NotesJain BabuNo ratings yet
- K 223387 FdaDocument12 pagesK 223387 Fdabashir019No ratings yet
- MODULE 1 Overview of The InstitutionDocument45 pagesMODULE 1 Overview of The InstitutionShannen CostoNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document12 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993nlongsisvnNo ratings yet
- Department of Health & Human ServicesDocument10 pagesDepartment of Health & Human ServicesLyht TVNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document24 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Vruddhi BhatiaNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document10 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993infoabhaypNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document19 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Aagam ShahNo ratings yet
- Maxima FDA ApprovalDocument10 pagesMaxima FDA ApprovalAla'a IsmailNo ratings yet
- Department of Health & Human ServicesDocument10 pagesDepartment of Health & Human ServicesFaty ShekoohiNo ratings yet
- PM G 20120301Document52 pagesPM G 20120301serruchogbNo ratings yet
- BDMax EntericViralPanel PDFDocument24 pagesBDMax EntericViralPanel PDFTNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Sami MoqbelNo ratings yet
- K210699 Resona I9Document10 pagesK210699 Resona I9Mohammed AliNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document15 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Phạm Quốc AnhNo ratings yet
- Fda Advanced 2017Document7 pagesFda Advanced 2017Servicio Tecnico Biomedico AsociadoNo ratings yet
- Case Study and Reading Control Form: Documentation Template & Evaluation InstrumentDocument5 pagesCase Study and Reading Control Form: Documentation Template & Evaluation InstrumentKonisbell Alcántara UreñaNo ratings yet
- K 171580Document10 pagesK 171580DavidNo ratings yet
- 5 10 (K) SUMMARY: Contact Person:FDDocument5 pages5 10 (K) SUMMARY: Contact Person:FDHadanNo ratings yet
- Department of Health & Human ServicesDocument5 pagesDepartment of Health & Human ServicessdwNo ratings yet
- Data Digitization and DisruptionDocument18 pagesData Digitization and DisruptionSimpleLuNo ratings yet
- Equalance Comparission DataDocument41 pagesEqualance Comparission DataAnil Chowadary Anil ChowadaryNo ratings yet
- Outsourcing Technology In the Healthcare Industry: In Depth Research to Protect the Security, Technology, and Profitability of Your BusinessFrom EverandOutsourcing Technology In the Healthcare Industry: In Depth Research to Protect the Security, Technology, and Profitability of Your BusinessNo ratings yet
- To Do CurrentDocument10 pagesTo Do Currentapi-894731No ratings yet
- To Do CurrentDocument5 pagesTo Do Currentapi-894731No ratings yet
- Register Blood Bank Scenario v4 Blood Topic PageDocument5 pagesRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page No Left NavDocument1 pageBiologics Topic Page No Left Navapi-894731No ratings yet
- Donating Blood Page No CalloutsDocument1 pageDonating Blood Page No Calloutsapi-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- CVM WireframesDocument4 pagesCVM Wireframesapi-894731No ratings yet
- Giving Blood Scenario v2Document3 pagesGiving Blood Scenario v2api-894731No ratings yet
- Register Blood Bank Scenario v2Document4 pagesRegister Blood Bank Scenario v2api-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page OnlyDocument5 pagesBiologics Topic Page Onlyapi-894731No ratings yet
- Importance of DincharyaDocument3 pagesImportance of DincharyaIOSRjournalNo ratings yet
- Foster Care Case StudyDocument6 pagesFoster Care Case StudyErica TuthillNo ratings yet
- Minor Project Plastic WasteDocument24 pagesMinor Project Plastic Wastearjun kumar0% (1)
- Math 8-Sum and Diff of RAE. Solving Different DenominatorDocument30 pagesMath 8-Sum and Diff of RAE. Solving Different DenominatorRegine AranzaNo ratings yet
- Clinical Research Brochure PrintDocument2 pagesClinical Research Brochure PrintSandeep ReddyNo ratings yet
- Perspectives of New Music. Brian Ferneyhough in Conversation With Jame BorosDocument46 pagesPerspectives of New Music. Brian Ferneyhough in Conversation With Jame BorosCharlex López100% (3)
- 5 Enote 5Document81 pages5 Enote 5Huzefa shaikhNo ratings yet
- What Is Oracle FinancialsDocument262 pagesWhat Is Oracle FinancialsSrimannarayana KasthalaNo ratings yet
- A Two: Photography in Anthropology: On ExperimentsDocument17 pagesA Two: Photography in Anthropology: On ExperimentsRelja PekićNo ratings yet
- A New Method Development and Validation of Axitinib Bulk and Pharmaceutical Dosage Form by Usinguv-Visible Spectroscopy As Per Ich GuidelinesDocument7 pagesA New Method Development and Validation of Axitinib Bulk and Pharmaceutical Dosage Form by Usinguv-Visible Spectroscopy As Per Ich GuidelinesBaru Chandrasekhar RaoNo ratings yet
- CDH4 Installation GuideDocument324 pagesCDH4 Installation GuideArif CupuNo ratings yet
- Machine Design SyllabusDocument2 pagesMachine Design SyllabusssjNo ratings yet
- Class 7Document17 pagesClass 7Roli DubeNo ratings yet
- NOSPlanDocument10 pagesNOSPlanDisha MendhekarNo ratings yet
- Factor That Influence PerceptionDocument5 pagesFactor That Influence PerceptionZikkru ThaqibNo ratings yet
- Proscan35E Prospektblatt EDocument3 pagesProscan35E Prospektblatt EDiego SalazarNo ratings yet
- An Introduction To Dead Weight TestersDocument2 pagesAn Introduction To Dead Weight TestersAsep HermanNo ratings yet
- GengarDocument27 pagesGengarshivan30No ratings yet
- BS - Term 1 - October 2022 - Dr. TS - 6CM - QPDocument2 pagesBS - Term 1 - October 2022 - Dr. TS - 6CM - QPShivam KumarNo ratings yet
- Cognitive Rigidity - The 8-Ball From HellDocument3 pagesCognitive Rigidity - The 8-Ball From HellNicasio AquinoNo ratings yet
- 1987 UNEP Goals and Principles of Environmental Impact AssessmentDocument2 pages1987 UNEP Goals and Principles of Environmental Impact AssessmentjouchanNo ratings yet
- Types of Slope ProtectionDocument40 pagesTypes of Slope ProtectionLouies Ungria100% (3)
- 2022-03-15 Reference ListDocument2 pages2022-03-15 Reference ListLa TaNo ratings yet
- Almost Final DraftDocument5 pagesAlmost Final Draftapi-271906472No ratings yet
Manufacturer Page Rewritten - HTM
Manufacturer Page Rewritten - HTM
Uploaded by
api-894731Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Manufacturer Page Rewritten - HTM
Manufacturer Page Rewritten - HTM
Uploaded by
api-894731Copyright:
Available Formats
Text Size Notes:
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
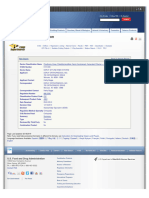
1 This is a rewrite of the text on the Industry page: http://www.fda.gov/cber/
manufacturer.htm.
Biologics, Blood & Vaccines
The Industry page refers to both the “Manufacturers Assistance Branch” and the
“Manufacturers Assistance and Technical Training Branch”. I didn’t know which name
Biologics, Blood & Vaccines was current, so I used the shorter of the two in this wireframe.
Biologics, Blood &
Vaccines The page now has an announcement for a training program that closed on February 21.
Guidance, I did not include this announcement in this wireframe.
Guidance, Compliance & Regulatory Information
Compliance &
The Manufacturers Assistance Branch can answer questions on many policy and Email Page
Regulatory procedural topics including: 2 These are the same general phone numbers shown in the Contact Us module. Are there
Information o Information on clinical investigators Print Page more specific numbers to use?
1
o How to report an adverse event
Bookmark and share
Acts, Rules & o How to submit an application online (electronic submission) Is the e-mail address an individual’s name or an acronym for the Manufacturers
Regulations o How to submit an Investigational New Drug Application (IND) to administer Get email updates Assistance and Technical Training Branch? If the latter, can it be in all CAPS, so it will
an investigational product to humans
Subscribe to RSS look more like an acronym?
Guidances Please contact us:
o Phone: 800-835-4709 or 301-827-1800 2
Establishment o E-mail: matt@cber.fda.gov
Registration
We answer questions and provide training to:
o Large and small manufacturers
Compliance Activities o Trade associations
Enforcement
Acts, Rules & Regulations Contact Us
Post-Market Activities Public Health Service Act 800-835-4709
301-827-1800
Rules
Imports & Exports
Comprehensive List of Laws Enforced octma@cber.fda.gov
Code of Federal Regulations – Biologics Enforced Center for Biologics
Evaluation and Research
1401 Rockville Pike, Suite
Guidances & SOPs 200N
Rockville, MD 20852-1448
Guidances by Topic & Year
Resources for You Manual of SOPs Media Inquiries
Consumers & Healthcare About CBER
Providers Imports & Exports
Compliance Program Guidance Manual – Imported CBER-Regulated
Industry
Products
Export Certificates
Importing Samples for Research Use Only
Other Topics
Compliance Activities
Enforcement
Post-Market Activities
Page Last Reviewed:
Page Last Updated:
Content Source:
Project: FDA Template Wireframes
FDA Footer Prepared By: Cari A. Wolfson, Focus on U!
Date Modified: 10/28/2008 by Design for
Context
Guidance List Page - CWp22 Page Number: 1 of 1
You might also like
- Example of PM Interview PresentationDocument12 pagesExample of PM Interview PresentationAlex Gomez0% (1)
- SILC Working DraftDocument21 pagesSILC Working DraftBar & BenchNo ratings yet
- Stress Analysis Convergence Tips For - Dummies - CAE AssociatesDocument4 pagesStress Analysis Convergence Tips For - Dummies - CAE Associatessukhabhukha987No ratings yet
- Swim Ultra Efficient Freestyle WorkbookDocument140 pagesSwim Ultra Efficient Freestyle WorkbookMichel D100% (12)
- LAB 5 - Pineapple CordialsDocument17 pagesLAB 5 - Pineapple Cordialsghostly_form416995% (21)
- Giving Blood Scenario v2Document3 pagesGiving Blood Scenario v2api-894731No ratings yet
- Biologics Topic Page OnlyDocument5 pagesBiologics Topic Page Onlyapi-894731No ratings yet
- k162919 Device DescriptionDocument7 pagesk162919 Device DescriptionjsdanielinNo ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Digital Health Marketplace Summary For Canada 1635193267Document28 pagesDigital Health Marketplace Summary For Canada 1635193267arushi166No ratings yet
- Register Blood Bank Scenario v2Document4 pagesRegister Blood Bank Scenario v2api-894731No ratings yet
- FDA Easy on-PCDocument5 pagesFDA Easy on-PCVictor CuellarNo ratings yet
- FDA SofwaveDocument9 pagesFDA SofwavePhúc LâmNo ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Latin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)Document4 pagesLatin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)ComplianceOnlineNo ratings yet
- 2.2 (Rie Matsui - Eng) Vietnam WS (19oct2023)Document25 pages2.2 (Rie Matsui - Eng) Vietnam WS (19oct2023)tovanbac96No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Fda K161533Document11 pagesFda K161533Оксана КожокаруNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993ryan resultsNo ratings yet
- Small Business Qualification Certification GuidanceDocument31 pagesSmall Business Qualification Certification GuidanceNataliaNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document9 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Manoj NarukaNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993liuyonglogNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document11 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Gerardo DiazNo ratings yet
- USFundamentals Chapter36Document21 pagesUSFundamentals Chapter36Phan Do Dang KhoaNo ratings yet
- LaseMD LEO Laser System FDADocument9 pagesLaseMD LEO Laser System FDAakamteb.rdNo ratings yet
- K232431 - Brain Hemmorage - SiemensDocument8 pagesK232431 - Brain Hemmorage - Siemensmario.wisconsinNo ratings yet
- 510KDocument11 pages510Ksandy zhengNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document6 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993venkat_bhagavatiNo ratings yet
- 2019 IPI Winter Web CompressedDocument92 pages2019 IPI Winter Web CompressedKamran AlamNo ratings yet
- U .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Document5 pagesU .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Larissa GolucciNo ratings yet
- Solving Pharmas Quality Unit Identity CrisisDocument3 pagesSolving Pharmas Quality Unit Identity CrisisAYMEN GOODKidNo ratings yet
- Arkray Adams A1c Lite HA-8380V - FDA Datasheets and InterferenceDocument28 pagesArkray Adams A1c Lite HA-8380V - FDA Datasheets and Interferencemidifast2aNo ratings yet
- K 160412Document22 pagesK 160412Louelle HopeNo ratings yet
- Dia #1Document12 pagesDia #1Gaxi BofNo ratings yet
- 510 (K) Premarket NotificationDocument1 page510 (K) Premarket NotificationkuttyjNo ratings yet
- Register Blood Bank Scenario v4 Blood Topic PageDocument5 pagesRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731No ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document9 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Hau TyanNo ratings yet
- Helping Pharmas Manage Compliance Risks For Speaker ProgramsDocument9 pagesHelping Pharmas Manage Compliance Risks For Speaker ProgramsCognizantNo ratings yet
- Argos 510KDocument10 pagesArgos 510KNorman GuntsonNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993QFCarlosCQNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document13 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993ryan resultsNo ratings yet
- PD-400424 Rev B HolterCare 11.0.1 Release NotesDocument8 pagesPD-400424 Rev B HolterCare 11.0.1 Release NotesJain BabuNo ratings yet
- K 223387 FdaDocument12 pagesK 223387 Fdabashir019No ratings yet
- MODULE 1 Overview of The InstitutionDocument45 pagesMODULE 1 Overview of The InstitutionShannen CostoNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document12 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993nlongsisvnNo ratings yet
- Department of Health & Human ServicesDocument10 pagesDepartment of Health & Human ServicesLyht TVNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document24 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Vruddhi BhatiaNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document10 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993infoabhaypNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document19 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Aagam ShahNo ratings yet
- Maxima FDA ApprovalDocument10 pagesMaxima FDA ApprovalAla'a IsmailNo ratings yet
- Department of Health & Human ServicesDocument10 pagesDepartment of Health & Human ServicesFaty ShekoohiNo ratings yet
- PM G 20120301Document52 pagesPM G 20120301serruchogbNo ratings yet
- BDMax EntericViralPanel PDFDocument24 pagesBDMax EntericViralPanel PDFTNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Sami MoqbelNo ratings yet
- K210699 Resona I9Document10 pagesK210699 Resona I9Mohammed AliNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document15 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Phạm Quốc AnhNo ratings yet
- Fda Advanced 2017Document7 pagesFda Advanced 2017Servicio Tecnico Biomedico AsociadoNo ratings yet
- Case Study and Reading Control Form: Documentation Template & Evaluation InstrumentDocument5 pagesCase Study and Reading Control Form: Documentation Template & Evaluation InstrumentKonisbell Alcántara UreñaNo ratings yet
- K 171580Document10 pagesK 171580DavidNo ratings yet
- 5 10 (K) SUMMARY: Contact Person:FDDocument5 pages5 10 (K) SUMMARY: Contact Person:FDHadanNo ratings yet
- Department of Health & Human ServicesDocument5 pagesDepartment of Health & Human ServicessdwNo ratings yet
- Data Digitization and DisruptionDocument18 pagesData Digitization and DisruptionSimpleLuNo ratings yet
- Equalance Comparission DataDocument41 pagesEqualance Comparission DataAnil Chowadary Anil ChowadaryNo ratings yet
- Outsourcing Technology In the Healthcare Industry: In Depth Research to Protect the Security, Technology, and Profitability of Your BusinessFrom EverandOutsourcing Technology In the Healthcare Industry: In Depth Research to Protect the Security, Technology, and Profitability of Your BusinessNo ratings yet
- To Do CurrentDocument10 pagesTo Do Currentapi-894731No ratings yet
- To Do CurrentDocument5 pagesTo Do Currentapi-894731No ratings yet
- Register Blood Bank Scenario v4 Blood Topic PageDocument5 pagesRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Combination Topic Page OnlyDocument1 pageCombination Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page No Left NavDocument1 pageBiologics Topic Page No Left Navapi-894731No ratings yet
- Donating Blood Page No CalloutsDocument1 pageDonating Blood Page No Calloutsapi-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- CVM WireframesDocument4 pagesCVM Wireframesapi-894731No ratings yet
- Giving Blood Scenario v2Document3 pagesGiving Blood Scenario v2api-894731No ratings yet
- Register Blood Bank Scenario v2Document4 pagesRegister Blood Bank Scenario v2api-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page OnlyDocument5 pagesBiologics Topic Page Onlyapi-894731No ratings yet
- Importance of DincharyaDocument3 pagesImportance of DincharyaIOSRjournalNo ratings yet
- Foster Care Case StudyDocument6 pagesFoster Care Case StudyErica TuthillNo ratings yet
- Minor Project Plastic WasteDocument24 pagesMinor Project Plastic Wastearjun kumar0% (1)
- Math 8-Sum and Diff of RAE. Solving Different DenominatorDocument30 pagesMath 8-Sum and Diff of RAE. Solving Different DenominatorRegine AranzaNo ratings yet
- Clinical Research Brochure PrintDocument2 pagesClinical Research Brochure PrintSandeep ReddyNo ratings yet
- Perspectives of New Music. Brian Ferneyhough in Conversation With Jame BorosDocument46 pagesPerspectives of New Music. Brian Ferneyhough in Conversation With Jame BorosCharlex López100% (3)
- 5 Enote 5Document81 pages5 Enote 5Huzefa shaikhNo ratings yet
- What Is Oracle FinancialsDocument262 pagesWhat Is Oracle FinancialsSrimannarayana KasthalaNo ratings yet
- A Two: Photography in Anthropology: On ExperimentsDocument17 pagesA Two: Photography in Anthropology: On ExperimentsRelja PekićNo ratings yet
- A New Method Development and Validation of Axitinib Bulk and Pharmaceutical Dosage Form by Usinguv-Visible Spectroscopy As Per Ich GuidelinesDocument7 pagesA New Method Development and Validation of Axitinib Bulk and Pharmaceutical Dosage Form by Usinguv-Visible Spectroscopy As Per Ich GuidelinesBaru Chandrasekhar RaoNo ratings yet
- CDH4 Installation GuideDocument324 pagesCDH4 Installation GuideArif CupuNo ratings yet
- Machine Design SyllabusDocument2 pagesMachine Design SyllabusssjNo ratings yet
- Class 7Document17 pagesClass 7Roli DubeNo ratings yet
- NOSPlanDocument10 pagesNOSPlanDisha MendhekarNo ratings yet
- Factor That Influence PerceptionDocument5 pagesFactor That Influence PerceptionZikkru ThaqibNo ratings yet
- Proscan35E Prospektblatt EDocument3 pagesProscan35E Prospektblatt EDiego SalazarNo ratings yet
- An Introduction To Dead Weight TestersDocument2 pagesAn Introduction To Dead Weight TestersAsep HermanNo ratings yet
- GengarDocument27 pagesGengarshivan30No ratings yet
- BS - Term 1 - October 2022 - Dr. TS - 6CM - QPDocument2 pagesBS - Term 1 - October 2022 - Dr. TS - 6CM - QPShivam KumarNo ratings yet
- Cognitive Rigidity - The 8-Ball From HellDocument3 pagesCognitive Rigidity - The 8-Ball From HellNicasio AquinoNo ratings yet
- 1987 UNEP Goals and Principles of Environmental Impact AssessmentDocument2 pages1987 UNEP Goals and Principles of Environmental Impact AssessmentjouchanNo ratings yet
- Types of Slope ProtectionDocument40 pagesTypes of Slope ProtectionLouies Ungria100% (3)
- 2022-03-15 Reference ListDocument2 pages2022-03-15 Reference ListLa TaNo ratings yet
- Almost Final DraftDocument5 pagesAlmost Final Draftapi-271906472No ratings yet