Professional Documents
Culture Documents
Thermochemistry
Thermochemistry
Uploaded by
rskr_tOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermochemistry
Thermochemistry
Uploaded by
rskr_tCopyright:
Available Formats
Thermodynamics - I
Thermodynamics is the science of the relationships between heat and other forms of energy. Thermochemistry is one area of thermodynamics. It concerns the study of the quantity of heat absorbed or evolved (given off) by chemical reactions.
Energy
We can define energy briefly as the potential or capacity to move matter. According to this definition, energy is not a material thing but rather a property of matter. The calorie (cal) is a non-SI unit of energy commonly used by chemists, originally defined as the amount of energy required to raise the temperature of one gram of water by one degree Celsius. Kinetic energy is the energy associated with an object by virtue of its motion. Potential energy is the energy an object has by virtue of its position in a field of force. The sum of the kinetic and potential energies of the particles making up a substance is referred to as the internal energy, U, of the substance. Therefore, the total energy, Etot, of a quantity of water equals the sum of its kinetic and potential energies as a whole (Ek + Ep) plus its internal energy. Etotal = U + Ek + Ep
Law of Conservation of Energy
Energy may be converted from one form to another, but the total quantity of energy remains constant.
System
The substance or mixture of substances under study in which a change occurs is called the thermodynamic system (or simply system). The surroundings are everything in the vicinity of the thermodynamic system.
Heat
Heat is defined as the energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings. As long as a system and its surroundings are in thermal contact (that is, they are not thermally insulated from one another), energy (heat) flows between them to establish temperature equality, or thermal equilibrium. Heat flows from a region of higher temperature to one of lower temperature; once the temperatures become equal, heat flow stops. Imagine two vessels in contact, each containing oxygen gas and the one on the left being hotter. According to kinetic theory, the average speed of molecules in the hotter gas is greater than that of molecules in the colder gas. But as the molecules in their random motions collide with the vessel walls, they lose energy to or gain energy from the walls. The faster molecules tend to slow down, while the slower molecules tend to speed up. Eventually, the average speeds of the molecules in the two vessels (and therefore the temperatures of the two gases) become equal. The net result is that energy is transferred through the vessel walls from the hot gas to the cold gas; that is, heat has flowed from the hotter vessel to the cooler one. Heat is denoted by the symbol q. The algebraic sign of q is chosen to be positive if heat is absorbed by the system and negative if heat is evolved. The sign of q can be
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674 2531733
Thermodynamics - I remembered this way: when heat is absorbed by a system, energy is added to it; q is assigned a positive quantity. On the other hand, when heat is evolved by a system, energy is subtracted from it; q is assigned a negative number.
Heat of reaction
The heat of reaction (at a given temperature) is the value of q required to return a system to the given temperature at the completion of the reaction. Chemical reactions or physical changes are classified as exothermic or endothermic. An exothermic process is a chemical reaction or a physical change in which heat is evolved (Q is negative). An endothermic process is a chemical reaction or a physical change in which heat is absorbed (Q is positive).
Enthalpy
Enthalpy (denoted H) is an extensive property of a substance that can be used to obtain the heat absorbed or evolved in a chemical reaction. (An extensive property is a property that depends on the amount of substance. Other examples of extensive properties are mass and volume.) Enthalpy is related to the heat of reaction qp. Enthalpy is a state function. A state function is a property of a system that depends only on its present state, which is determined by variables such as temperature and pressure, and is independent of any previous history of the system.
Enthalpy of Reaction(Hr)
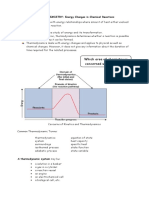
The change in enthalpy for a reaction at a given temperature and pressure (called the enthalpy of reaction). Because H is a state function, the value of H is independent of the details of the reaction. It depends only on the initial state (the reactants) and the final state (the products). This is obtained by subtracting the enthalpy of the reactants from the enthalpy of the products. H = H(products) - H(reactants) The enthalpy of reaction equals the heat of reaction at constant pressure. To illustrate this, consider the reaction at 25 C of sodium metal and water, carried out in a beaker open to the atmosphere at 1.00 atm pressure. 2Na(s) + 2H2O(l ) 2NaOH(aq) + H2(g) The metal and water react vigorously and heat evolves. Experiment shows that 2 mol of sodium metal reacts with 2 mol of water to evolve 368.6 kJ of heat. Because heat evolves, the reaction is exothermic, and you write H = -368.6 kJ. Figure shows an enthalpy diagram for this reaction.
Enthalpy and Internal Energy
We define enthalpy, H, precisely as the quantity U + PV. Because U, P, and V are state functions, H is also a state function. This means that for a given temperature and pressure, a given amount of a substance has a definite enthalpy. Therefore, if you know the enthalpies of substances, you can calculate the change of enthalpy, H, for a reaction. Note that H is the final enthalpy Hf minus the initial enthalpy Hi. H = Hf - Hi At constant pressure
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674-2531733
Thermodynamics - I H = (Uf + PVf) - (Ui + PVi) = (Uf - Ui) + P(Vf - Vi) = U + PV By rearranging we get U = H - PV The last term in this equation (-PV) is the energy required by the system to change volume against the constant pressure of the atmosphere. (The minus sign means that energy is required to increase the volume of the system.) This required energy is called the pressurevolume work.
Thermochemical equation
A thermochemical equation is the chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written after the equation. For the reaction of sodium and water, you would write 2Na(s) + 2H2O(l ) 2NaOH(aq) + H2(g); H = -368.6 kJ This equation says that 2 mol of sodium reacts with 2 mol of water to produce 2 mol of sodium hydroxide and 1 mol of hydrogen gas, and 368.6 kJ of heat evolves. Note that the thermochemical equation includes phase labels. This is because the enthalpy change, H, depends on the phase of the substances. The following are two important rules for manipulating thermochemical equations: 1. Enthalpy is an extensive property. Hence when a thermochemical equation is multiplied by any factor, the value of H for the new equation is obtained by multiplying the value of H in the original equation by that same factor. 2. Enthalpy is a state function. Thus when a chemical equation is reversed, the value of H is reversed in sign.(Laplace and Lavoisiers law)
Heat Capacity and Specific Heat
The heat capacity (C) of a sample of substance is the quantity of heat needed to raise the temperature of the sample of substance one degree Celsius (or one kelvin). q = CT The specific heat capacity (or simply specific heat) is the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one kelvin) at constant pressure. To find the heat q required to raise the temperature of a sample, you multiply the specific heat of the substance, c, by the mass in grams, m, and the temperature change, T. q = mcT A calorimeter, a device used to measure the heat absorbed or evolved during a physical or chemical change.
Hesss Law of Constant Heat Summation
Hesss law of constant heat summation states that for a chemical equation that can be written as the sum of two or more steps, the enthalpy change for the overall equation equals the sum of the enthalpy changes for the individual steps. Enthalpy is a state function. This means that the enthalpy change for a chemical reaction is independent of the path by which the products are obtained. To understand Hesss law and to see how you can use it, consider a simple example. Suppose you would like to find the enthalpy change for the combustion of graphite (carbon) to carbon monoxide. 2C(graphite) + O2(g) 2CO(g) The direct determination of this enthalpy change is very difficult, because once carbon monoxide forms it reacts further with oxygen to yield carbon dioxide. If you do the experiment in an excess of oxygen, you obtain only carbon dioxide, and the enthalpy
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674-2531733
Thermodynamics - I change is the heat of complete combustion of graphite. On the other hand, if you do the experiment in a limited quantity of oxygen, you obtain a mixture of carbon monoxide and carbon dioxide, and the heat of reaction is a value appropriate for a mixture of these products. How can you obtain the enthalpy change for the preparation of pure carbon monoxide from graphite and oxygen? The answer is to apply Hesss law. To do this, imagine that the combustion of graphite to carbon monoxide takes place in two separate steps: 2C(graphite) + 2O2(g) 2CO2(g) (first step) 2CO2(g) 2CO(g) + O2(g) (second step) In the first step, you burn 2 mol of graphite in 2 mol of oxygen to produce 2 mol of carbon dioxide. In the second step, you decompose this carbon dioxide to give 2 mol of carbon monoxide and 1 mol of oxygen. The net result is the combustion of 2 mol of graphite in 1 mol of oxygen to give 2 mol of carbon monoxide. You can obtain this result by adding the two steps, canceling out 2 mol CO2 and 1 mol O2 on both sides of the equation. 2C(graphite) + 2O2(g) 2CO2(g) 2CO2(g) 2CO(g) + O2(g) 2C(graphite) + O2(g) 2CO(g) According to Hesss law, the enthalpy change for the overall equation (which is the equation you want) equals the sum of the enthalpy changes for the two steps. Now you need to determine the enthalpy changes for the separate steps. You can determine the enthalpy change for the first step by simply burning graphite in an excess of oxygen. The result is H = -393.5 kJ per mole of CO2 formed. For 2 mol CO2, you multiply by 2. 2C(graphite) +2O2(g) 2CO2(g); H = (-393.5 kJ) x 2 The second step, the decomposition of carbon dioxide, is not an easy experiment. However, the reverse of this decomposition is simply the combustion of carbon monoxide. You could determine the H for that combustion by burning carbon monoxide in an excess of oxygen. The experiment is similar to the one for the combustion of graphite to carbon dioxide. 2CO(g) + O2(g) 2CO2(g); H = -566.0 kJ From the properties of thermochemical equations, you know that the enthalpy change for the reverse reaction is simply -1 times the original reaction. 2CO2(g) 2CO(g) + O2(g); H = (-566.0 kJ) x(-1) If you now add these two steps and add their enthalpy changes, you obtain the chemical equation and the enthalpy change for the combustion of carbon monoxide, which is what you wanted. 2C(graphite) + 2O2(g) 2CO2(g) 2C(graphite) + O2(g) 2CO2(g) 2CO(g) + O2(g) 2CO(g) H1 = (-393.5 kJ) x 2 H2 = (-566 kJ) x (-1) H = H1 + H2 = -221 kJ
Figure gives an enthalpy diagram showing the relationship among the enthalpy changes for this calculation. The diagram shows two different ways to go from graphite and oxygen (reactants) to carbon monoxide (products). Going by way of reactions 1 and 2 is equivalent to the direct reaction 3.
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674-2531733
Thermodynamics - I
Standard Enthalpies of Formation
The term standard state refers to the standard thermodynamic conditions chosen for substances when listing or comparing thermodynamic data: 1 atm pressure and the specified temperature (usually 25C). These standard conditions are indicated by a superscript degree sign (). The enthalpy change for a reaction in which reactants in their standard states yield products in their standard states is denoted H (delta H degree, but often read as delta H zero). The quantity H is called the standard enthalpy of reaction. The elements oxygen and carbon are said to exist in different allotropic forms. An allotrope is one of two or more distinct forms of an element in the same physical state. The reference form of an element for the purpose of specifying the formation reaction is usually the stablest form (physical state and allotrope) of the element under standard thermodynamic conditions. The standard enthalpy of formation (also called the standard heat of formation) of a substance, denoted Hf, is the enthalpy change for the formation of one mole of the substance in its standard state from its elements in their reference form and in their standard states. In general, you can calculate the H for a reaction by the equation H = n Hf (products) - m Hf (reactants) Here is the mathematical symbol meaning the sum of, and m and n are the coefficients of the substances in the chemical equation.
Standard definitions and Enthalpy Nomenclatures:
Enthalpy type(molar enthalpies) Enthalpy of formation Examples Na(s) + Cl2(g) NaCl(s) CH4(g) + 2O2(g) 2H2O(l) H2O(s) H2O(l) H2O(s) CH4(g) H2O(l) H2O(g) H2O(g) C(g) + 4H(g) CO2(g) + Definitions One mole of compound formed from its elements, all reagents in standard states One mole of compound is completely burnt in oxygen, all reagents in standard states One mole of liquid if formed from the solid at constant temperature One mole of gas if formed from the liquid at constant temperature One mole of gas if formed from the solid at constant temperature One mole of substance is broken into atoms in gas phase One mole of water is formed by neutralization of an acid with a base One mole of atoms ionized, all species in gaseous phase
Enthalpy of combustion
Enthalpy of fusion Enthalpy of vaporization Enthalpy of sublimation Enthalpy of atomization Enthalpy of neutralization Enthalpy of ionization
HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Na(g) Na+(g) + e-(g)
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674-2531733
Thermodynamics - I Electron gain enthalpy Lattice enthalpy Bond dissociation enthalpy Enthalpy of solution Enthalpy of hydration Cl(g) + e- (g) NaCl(s) HCl(g) Cl-(g) One mole of anion formed by gaining electrons, all species in gaseous phase One mole of crystal broken into individual ions in gaseous phase One mole of bonds broken, all species in gaseous phase One mole of substance is dissolved in a solvent till the specified concentration One mole of ion in gaseous phase is hydrated
Na+(g) + Cl-(g) H(g) + Cl(g) X(solution)
X(s) + solvent X (g) X (aq)
Reversible & Irreversible Processes
Motions of many-particle systems, especially in the domain of thermostatics, are called processes. Central to thermostatics is the distinction between reversible processes, such as the flight of a thrown stone, and irreversible processes, such as the aforementioned tumbling rock. Irreversible processes are all those processes in which friction and its generalizations play a role. They are those which increase the sharing or mixing of energy. Irreversible processes, in the sense in which the term is used in thermostatics, transform macroscopic motion into the disorganized motion of all the small microscopic components involved: they increase the sharing and mixing of energy. Irreversible processes are therefore not strictly irreversible but their reversal is extremely improbable. Chemical reactions often seem to stop before they are complete. Such reactions are reversible. That is, the original reactants form products, but then the products react with themselves to give back the original reactants. A reversible change in thermodynamics is a change that can be reversed by an infinitesimal modification of a variable. The key word 'infinitesimal' sharpens the everyday meaning of the word 'reversible' as something that can change direction. We say that a system is in equilibrium with its surroundings if an infinitesimal change in the conditions in opposite directions results in opposite changes in its state. Suppose a gas is confined by a piston and that the external pressure, P is set equal to the pressure, p, of the confined gas. Such a system is in mechanical equilibrium with its surroundings because an infinitesimal change in the external pressure in either direction causes changes in volume in opposite directions. If the external pressure is reduced infinitesimally, then the gas expands slightly. If the external pressure is increased infinitesimally, then the gas contracts slightly. In either case the change is reversible in the thermodynamic sense. If, on the other hand, the external pressure differs measurably from the internal pressure, then changing p., infinitesimally will not decrease it below the pressure of the gas, so will not change the direction of the process. Such a system is not in mechanical equilibrium with its surroundings and the expansion is thermodynamically irreversible.
SCHOOL OF MATHEMATICS AND SCIENCE A World of Numbers and Logic Visit Us at: www.somas.in mail us: sms@somas.in contact: 0674-2531733
You might also like
- Physical Biochemistry Lecture NotesDocument9 pagesPhysical Biochemistry Lecture Noteschc300No ratings yet
- APP Installation Detroit Diesel S2000 7SA2000 PDFDocument233 pagesAPP Installation Detroit Diesel S2000 7SA2000 PDFthanhhai31No ratings yet
- Chem 211-212 Hess's LawDocument17 pagesChem 211-212 Hess's LawRiff ShahNo ratings yet
- Eq o o o o Eq: ND RDDocument8 pagesEq o o o o Eq: ND RDEl-Sayed MohammedNo ratings yet
- CO3 ThermodynamicsDocument72 pagesCO3 ThermodynamicsRonna IturaldeNo ratings yet
- ThermochemistryDocument52 pagesThermochemistryBiddut DasNo ratings yet
- Heat of ReactionDocument43 pagesHeat of ReactionJohn Paul Bustante PlantasNo ratings yet
- Thermochemistry Summary - Libre TextsDocument4 pagesThermochemistry Summary - Libre Textsmacky 2No ratings yet
- DP ThermodynamicsDocument24 pagesDP ThermodynamicsYash AkhauriNo ratings yet
- Chapter 17 Outline Chem 1062: Probability To States of High ProbabilityDocument9 pagesChapter 17 Outline Chem 1062: Probability To States of High Probabilityaq300No ratings yet
- Chapter 4 CH 109Document24 pagesChapter 4 CH 109junaidNo ratings yet
- Thermochemistry Answers RemovedDocument11 pagesThermochemistry Answers Removedapi-327309463No ratings yet
- Duhok Polytechnic University Petrochemical Department Second Stage Physical ChemistryDocument6 pagesDuhok Polytechnic University Petrochemical Department Second Stage Physical Chemistrylya AhmedNo ratings yet
- Thermo ChemistryDocument11 pagesThermo ChemistryEugenTutunaruNo ratings yet
- CHAPTER 8 (References)Document10 pagesCHAPTER 8 (References)JeromeNo ratings yet
- ThermochemistryDocument5 pagesThermochemistryjoelsantos1981No ratings yet
- ThermochemistryDocument25 pagesThermochemistrydanielmahsaNo ratings yet
- Met Thermo 2Document10 pagesMet Thermo 2msahisriNo ratings yet
- Study ChemDocument13 pagesStudy ChemJanthina Rose AusteroNo ratings yet
- Chapter 6 Lecture NotesDocument10 pagesChapter 6 Lecture NotesAhmad KamalNo ratings yet
- Lecture 3 - Energy Changes in Chem RXNDocument50 pagesLecture 3 - Energy Changes in Chem RXNHedric VillenaNo ratings yet
- Enthalpy of Formation PDFDocument10 pagesEnthalpy of Formation PDFatulsemiloNo ratings yet
- CHM 101 Thermochemistry-1Document8 pagesCHM 101 Thermochemistry-1Olamide KoleNo ratings yet
- Chem 2Document82 pagesChem 2César ArenasNo ratings yet
- 8 1 Problem SetDocument11 pages8 1 Problem Setapi-182809945No ratings yet
- Genchem NotesDocument8 pagesGenchem NotesKarl BayaninNo ratings yet
- The Key: Thermochemistry Is The Branch of Physical Chemistry Which Deals With The Thermal or Heat ChangesDocument23 pagesThe Key: Thermochemistry Is The Branch of Physical Chemistry Which Deals With The Thermal or Heat ChangesSachin KumarNo ratings yet
- 3 ThermochemistryDocument11 pages3 Thermochemistryjosephmartinsogbu30No ratings yet
- P 7.4 ThermochemistryDocument32 pagesP 7.4 ThermochemistryFelicia GunawanNo ratings yet
- Intro 1a ThermochemistryDocument50 pagesIntro 1a ThermochemistryFatin IziantiNo ratings yet
- UntitledDocument16 pagesUntitledapi-233404189100% (1)
- Book Summary: A. The Nature of Energy and Types of EnergyDocument9 pagesBook Summary: A. The Nature of Energy and Types of EnergyFildzahNo ratings yet
- Hess Law NotesDocument13 pagesHess Law NotesMollin SiwellaNo ratings yet
- Energy - CHEM 106Document29 pagesEnergy - CHEM 106Eunice MaeNo ratings yet
- Reviewer ChemDocument17 pagesReviewer ChemJen Cyril Villadiego UntalanNo ratings yet
- Thermo ReviewDocument8 pagesThermo ReviewJosh CohenNo ratings yet
- LAS General Chemistry 2 Q4W12Document16 pagesLAS General Chemistry 2 Q4W12Marlon C. CambayNo ratings yet
- Chapter 19 Chemical ThermodynamicsDocument8 pagesChapter 19 Chemical ThermodynamicsRSLNo ratings yet
- Thermochemistry - Chemistry EncyclopediaDocument5 pagesThermochemistry - Chemistry EncyclopediaTwaine Angelle Villanueva SalvañaNo ratings yet
- THERMOODocument3 pagesTHERMOOKisha KhuranaNo ratings yet
- Chapter 17 - Thermodyamic QuantitiesDocument12 pagesChapter 17 - Thermodyamic QuantitiesAlvaro SotoNo ratings yet
- THERMODYNAMICSDocument3 pagesTHERMODYNAMICSAngelene Nova MondaresNo ratings yet
- Which Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsDocument19 pagesWhich Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsSahada KanapiyaNo ratings yet
- Unit 4: Thermochemistry and Nuclear Chemistry: Initial FinalDocument21 pagesUnit 4: Thermochemistry and Nuclear Chemistry: Initial FinalPankaj KumarNo ratings yet
- THERMOCHEMISTRY Hand Outs 2023Document6 pagesTHERMOCHEMISTRY Hand Outs 2023Paul Willard GumapacNo ratings yet
- Thermodynamic: Refresher MaterialDocument5 pagesThermodynamic: Refresher MaterialAnonymous dZ0UQedUiwNo ratings yet
- Laporan Kimia TermokimiaDocument17 pagesLaporan Kimia Termokimiashlynnn dyahNo ratings yet
- Lesson 8 ThermochemistryDocument38 pagesLesson 8 ThermochemistryLyndy PantaoNo ratings yet
- Ch06 SlidesDocument73 pagesCh06 SlidesbeelzeburtonNo ratings yet
- ThermochemistryDocument13 pagesThermochemistryDrazelle PangilinanNo ratings yet
- 7 Chapter 6Document58 pages7 Chapter 6azizNo ratings yet
- Energy Changes in Chemical Reactions Exothermic Reaction Is One That Releases Energy in The Form of Heat orDocument3 pagesEnergy Changes in Chemical Reactions Exothermic Reaction Is One That Releases Energy in The Form of Heat ormark bendanoNo ratings yet
- Thermodynamics & Chemical Equilibrium: Basic Geochemistry Teaching Team FTG 2017Document35 pagesThermodynamics & Chemical Equilibrium: Basic Geochemistry Teaching Team FTG 2017Goblin IkanNo ratings yet
- CH 101 Class 09 Energetics 01 PDFDocument12 pagesCH 101 Class 09 Energetics 01 PDFliz_hobbs79No ratings yet
- C6 ThermochemistryDocument57 pagesC6 ThermochemistryJhiGz Llausas de GuzmanNo ratings yet
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4From Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4No ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersNo ratings yet
- Trigo Viii & IxDocument2 pagesTrigo Viii & Ixrskr_tNo ratings yet
- Physics SyllabusDocument2 pagesPhysics Syllabusrskr_tNo ratings yet
- Page229 234Document6 pagesPage229 234rskr_tNo ratings yet
- Physical Chemistry Dr. R K Gupts Test - 02 Page No 293: Pale Yellow ColourlessDocument4 pagesPhysical Chemistry Dr. R K Gupts Test - 02 Page No 293: Pale Yellow Colourlessrskr_tNo ratings yet
- F-Dtn-M-Hfma Geology Paper-I: Time Allowed: Three Hours Maximum Marks: 300Document8 pagesF-Dtn-M-Hfma Geology Paper-I: Time Allowed: Three Hours Maximum Marks: 300rskr_tNo ratings yet
- More Than 5000 Questions Dr. BL Sharma Mathematics PageDocument4 pagesMore Than 5000 Questions Dr. BL Sharma Mathematics Pagerskr_tNo ratings yet
- Chemistry Test 02Document3 pagesChemistry Test 02rskr_tNo ratings yet
- Full Test 03Document4 pagesFull Test 03rskr_tNo ratings yet
- Introduction To ThermodynamicDocument7 pagesIntroduction To Thermodynamicrskr_tNo ratings yet
- Art 3Document3 pagesArt 3mohamedmosallamNo ratings yet
- Soal Google SketchupDocument2 pagesSoal Google SketchupNur Komaria100% (1)
- Self Healing RobotsDocument5 pagesSelf Healing RobotsshaluNo ratings yet
- Criminalization of Politics in India: A Project StudyDocument24 pagesCriminalization of Politics in India: A Project Studysanchitsingh75% (12)
- Doj It Strategic Plan 2022-2024Document26 pagesDoj It Strategic Plan 2022-2024Carlos CallejaNo ratings yet
- Modular ExponentiationDocument2 pagesModular ExponentiationflaksherNo ratings yet
- Grammar - Pre-Intermediate Progress Test Unit 9 Test A - PDF - Lee Harvey Oswald - Violence PDFDocument12 pagesGrammar - Pre-Intermediate Progress Test Unit 9 Test A - PDF - Lee Harvey Oswald - Violence PDFAnna PalšováNo ratings yet
- Bescom GSTN No: 29Aaccb1412G1Z5Document2 pagesBescom GSTN No: 29Aaccb1412G1Z5Ranjit AnandNo ratings yet
- Data Communication Errors: Networking 2Document24 pagesData Communication Errors: Networking 2Leonelyn Hermosa Gasco - CosidoNo ratings yet
- 1 Motor Protection Single SessionDocument27 pages1 Motor Protection Single Sessionmubarakkirko100% (1)
- Digital Electronics Lab ReportDocument4 pagesDigital Electronics Lab ReportMohammad Irfan Yousuf100% (1)
- Research Methods SESSIONS STUDENTS Abeeku PDFDocument287 pagesResearch Methods SESSIONS STUDENTS Abeeku PDFdomaina2008100% (3)
- RGPV SyllabusDocument9 pagesRGPV SyllabusNavneet PandharipandeNo ratings yet
- Consent vs. Coercion: BDSM Interactions Highlight A Fine But Immutable LineDocument10 pagesConsent vs. Coercion: BDSM Interactions Highlight A Fine But Immutable LineJulia WatanabeNo ratings yet
- NI 43 101 AntioquiaDocument205 pagesNI 43 101 AntioquiaKennet WoNo ratings yet
- UNIT-2-NOTES - Self Management Skills-IXDocument8 pagesUNIT-2-NOTES - Self Management Skills-IXVaishnavi JoshiNo ratings yet
- Trelleborg Elastomeric Bridge Bearings PDFDocument4 pagesTrelleborg Elastomeric Bridge Bearings PDFMad WonderNo ratings yet
- Data Modeling Entity Relationship DiagramsDocument27 pagesData Modeling Entity Relationship DiagramsPrachi SaxenaNo ratings yet
- EPDItaly Feralpi ColdRolled 281019Document15 pagesEPDItaly Feralpi ColdRolled 281019marcoNo ratings yet
- Kinematics: Distance Vs DisplacementDocument7 pagesKinematics: Distance Vs DisplacementTorettoNo ratings yet
- American Anthropologist - August 1963 - Aberle - Culture and Behavior Collected Essays of Clyde KluckhohnDocument3 pagesAmerican Anthropologist - August 1963 - Aberle - Culture and Behavior Collected Essays of Clyde KluckhohnJesicca PajaresNo ratings yet
- Case Study MMDocument3 pagesCase Study MMayam0% (1)
- How To Implement ABAP Kernel UpdateDocument4 pagesHow To Implement ABAP Kernel Updateabhisona76No ratings yet
- Chaltu FitaDocument84 pagesChaltu FitaÍťž Ñûř Ôřø ßœýNo ratings yet
- Mis 024Document223 pagesMis 024ysanyasirajuNo ratings yet
- Kubota Tractor B20 Loader Backhoe BT650 BT750 Operators ManualDocument32 pagesKubota Tractor B20 Loader Backhoe BT650 BT750 Operators ManualBob SeveranceNo ratings yet
- Et Iso 12543 3 2011Document10 pagesEt Iso 12543 3 2011freddyguzman3471No ratings yet
- Professional Project ManagementDocument2 pagesProfessional Project ManagementVirginia Virgie100% (3)
- Investigation of The Sympathetic Tripping Problem in Power Systems With Large Penetrations of Distributed GenerationDocument7 pagesInvestigation of The Sympathetic Tripping Problem in Power Systems With Large Penetrations of Distributed GenerationCaroline MeloNo ratings yet