Professional Documents
Culture Documents
230 S10 HW7
230 S10 HW7
Uploaded by
pumjlffoOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
230 S10 HW7
230 S10 HW7
Uploaded by
pumjlffoCopyright:
Available Formats
MSE 230
HW7 (due 03/04, 03/05)
Spring 2010
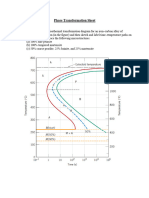
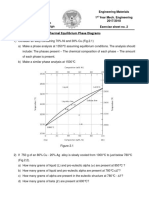
1. (a) Using the recrystallization kinetics data for pure copper in Fig. 10.11, what is the recrystallization temperature, defined as the temperature for which recrystallization is 95% complete in 1 h. (b) Briefly explain how and why the recrystallization temperature is affected by the following: i) solid solution impurities and ii) %CW. 2. Using the isothermal transformation diagram (Fig. 10.22) for eutectoid (1080) steel, determine the microstructure constituent(s) - Austenite (A), Pearlite (P), Bainite (B), Martensite (M) - and their approximate proportion(s) at the end of each of the following treatments. In each case the starting condition is Austentite at 800C. Assume all quenching is instantaneous. Assume any Martensite formed by quenching to room temperature contains 5% retained Austenite. (a) Quench to 600C and hold for 20 s, quench to room temperature. (b) Quench to 600C and hold for 7 s, quench to room temperature. (c) Quench to 175C and hold indefinitely. (d) Quench to 250C and hold for 1 min, quench to room temperature. (e) Quench to 250C and hold for 5 h, slow cool to room temperature. 3. Callister Problem 10.24: Figure 10.40 (below) shows the continuous cooling transformation diagram for a 0.35 wt.% C iron-carbon alloy. Sketch and label on the diagram continuous cooling curves to yield the following microstructures: (a) Fine pearlite and proeutectoid ferrite (b) Martensite (c) Martensite and proeutectoid ferrite (d) Coarse pearlite and proeutectoid ferrite (e) Marensite, fine pearlite and proeutectoid ferrite.
3. Rank order the structures (a)-(e) in Problem 3 from highest to lowest hardness and briefly justify your ranking by explaining the microstructural origins of the hardness differences. 4. Using Fig. 10.31 and the tempering data in Fig. 10.35, specify a tempering heat treatment (i.e., temperature and time) that when applied to the transformation product in problem 2(d) would result in the same hardness as transformation product in problem 2(e). 5. Sketch or trace the Al-rich end of the Al-Cu phase diagram in Fig. 11.24 out to about 10 wt.% Cu and to the right of it, using the same temperature axis scaling, make a quantitative temperature - time plot analogous to the schematic plot in Fig. 11.22, showing a precipitation hardening heat treatment sequence for an alloy containing 4.4 wt% Cu aged to peak (maximum) hardness at 204C. Justify your choice of the solution treatment temperature and use a solution treatment time of 1 h. Use the aging kinetics plot in Fig. 11.27. What would be the advantage of conducting the aging treatment at 150C? What would be the disadvantage?
You might also like
- Austenitizing Heat Treatment PDFDocument20 pagesAustenitizing Heat Treatment PDFsivajirao70100% (1)
- Hardenability of High CR White Cast IronDocument4 pagesHardenability of High CR White Cast IronanruloNo ratings yet
- Closed-Book Practice-Ch 10 (2017!08!08)Document12 pagesClosed-Book Practice-Ch 10 (2017!08!08)Juan0% (1)
- Phase Transformation SheetDocument3 pagesPhase Transformation Sheetmohamed.hassan031No ratings yet
- CH 11Document7 pagesCH 11Mario Misael Machado LòpezNo ratings yet
- Phase Transformation SheetDocument3 pagesPhase Transformation Sheetaz7303782No ratings yet
- MSE 260 Assignment 5Document2 pagesMSE 260 Assignment 5michael ananaNo ratings yet
- Time Temperature Transformation (TTT) Diagrams PDFDocument108 pagesTime Temperature Transformation (TTT) Diagrams PDFSerkan Apay100% (1)
- Phase TransformationDocument5 pagesPhase TransformationAlvin Tung Kwong Choong100% (1)
- PP Set 1 & 2Document3 pagesPP Set 1 & 2Raveendra kumar KosireddiNo ratings yet
- 1 PDFDocument1 page1 PDFYudi Candra BNo ratings yet
- 10.6 Continuous Cooling Transformation DiagramsDocument1 page10.6 Continuous Cooling Transformation DiagramsUlwan FianiNo ratings yet
- Quiz 1 G1 + ANSWERDocument3 pagesQuiz 1 G1 + ANSWERTayaChandranNo ratings yet
- Re Austenitisation MartensiteDocument4 pagesRe Austenitisation MartensiteAndress SsalomonnNo ratings yet
- ASSIGNMENT # 3 W KEYDocument16 pagesASSIGNMENT # 3 W KEYRashedul IslamNo ratings yet
- TDF - Assignment Week 12Document4 pagesTDF - Assignment Week 12Ayu Sekar TunjungNo ratings yet
- Capili Jefferson 11Document16 pagesCapili Jefferson 11Christian Al EncarnacionNo ratings yet
- Che 3330 - Spring 2012 HW 5Document5 pagesChe 3330 - Spring 2012 HW 5Brett CasserlyNo ratings yet
- Continuous Cooling Transformation (CCT) Diagrams: R. MannaDocument33 pagesContinuous Cooling Transformation (CCT) Diagrams: R. MannaYubefNo ratings yet
- Pearlite To AusteniteDocument11 pagesPearlite To AusteniteEnriqueMirandaGodoyNo ratings yet
- Sheet 2 (Material)Document6 pagesSheet 2 (Material)Omar AssalNo ratings yet
- Time Temperature Transformation With ReferncesDocument12 pagesTime Temperature Transformation With ReferncesEllie BrooklynNo ratings yet
- Assignment 1: DEADLINE: Monday, 17 January 2022Document10 pagesAssignment 1: DEADLINE: Monday, 17 January 2022Syafiqah RusdiNo ratings yet
- TTT Curves 1Document101 pagesTTT Curves 1ibrahimNo ratings yet
- R. Manna: Banaras Hindu UniversityDocument108 pagesR. Manna: Banaras Hindu UniversitypulilathaNo ratings yet
- CCT Diagram For Steels PDFDocument33 pagesCCT Diagram For Steels PDFGuru SamyNo ratings yet
- Homework 14 Solutions Spring 2001Document2 pagesHomework 14 Solutions Spring 2001Ikhwan Wf Miscellaneous AveroesNo ratings yet
- 1 s2.0 1359645496000845 MainDocument11 pages1 s2.0 1359645496000845 MainRaseem AhmedNo ratings yet
- Chapter 10Document4 pagesChapter 10Izzat NasrullahNo ratings yet
- Time Temperature Transformation With ReferncesDocument12 pagesTime Temperature Transformation With ReferncesEllie BrooklynNo ratings yet
- Untitled1 Capitulo10Document6 pagesUntitled1 Capitulo10Carlos CabanillasNo ratings yet
- SDMII Section8 Phases Transformation ExercisesDocument28 pagesSDMII Section8 Phases Transformation ExercisesPheng SeihaksethNo ratings yet
- Cementite DissolutionDocument12 pagesCementite DissolutionAdri SuryanaNo ratings yet
- 2018+Module+1+Term+Test+ SolutionsDocument8 pages2018+Module+1+Term+Test+ Solutionszubair ahmedNo ratings yet
- MSE 260 Assignment 6Document3 pagesMSE 260 Assignment 6michael ananaNo ratings yet
- ESO205 ProblemSheets-7 SolutionsDocument4 pagesESO205 ProblemSheets-7 SolutionsAjay chouhanNo ratings yet
- Part2 Heat Treatment of SteelDocument57 pagesPart2 Heat Treatment of SteelAhmed awwadNo ratings yet
- Tutorial 7 - Phase DiagramsDocument4 pagesTutorial 7 - Phase DiagramsSYAFIQAH ISMAILNo ratings yet
- TTT Phase DiagramDocument9 pagesTTT Phase Diagramhari krishnaNo ratings yet
- Publication 4 11889 199Document9 pagesPublication 4 11889 199Mulia AridhoNo ratings yet
- Iron Carbon Fe-C Equilibrium Diagram - An Iron Carbon EquilibriumDocument2 pagesIron Carbon Fe-C Equilibrium Diagram - An Iron Carbon Equilibriumروشان فاطمة روشانNo ratings yet
- Continuous Cooling Transformations in Nuclear Pressure Vessel SteelsDocument10 pagesContinuous Cooling Transformations in Nuclear Pressure Vessel SteelsVanina GiselaNo ratings yet
- 08 Transformasi Fasa - UASDocument35 pages08 Transformasi Fasa - UASDinta PratiwiNo ratings yet
- Formation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron RadiationDocument9 pagesFormation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron Radiationsmallik3No ratings yet
- Lichioiu IDocument6 pagesLichioiu ICristina MaierNo ratings yet
- Mat101 w12 Hw6 SolutionsDocument8 pagesMat101 w12 Hw6 SolutionsKonark PatelNo ratings yet
- Diagram FasaDocument6 pagesDiagram Fasaolid_zoneNo ratings yet
- r05320306 Heat TransferDocument8 pagesr05320306 Heat TransferSrinivasa Rao GNo ratings yet
- Effect of Austempering Time On Microstructure and Properties of A Low-Carbon Bainite SteelDocument7 pagesEffect of Austempering Time On Microstructure and Properties of A Low-Carbon Bainite Steelمسعود بوزويرNo ratings yet
- Chapter 8 (Heat Treatment)Document12 pagesChapter 8 (Heat Treatment)Shekinah KanindaNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument3 pagesIron-Iron Carbide Phase Diagram ExampleBenjamin Enmanuel Mango DNo ratings yet
- 01 - A CCT Diagram For An Offshore Pipeline Steel of X70 TypeDocument6 pages01 - A CCT Diagram For An Offshore Pipeline Steel of X70 TypeŞarîngă George AlexandruNo ratings yet
- Class Problem 10.24Document2 pagesClass Problem 10.24HarishNo ratings yet
- Aanca 2Document18 pagesAanca 2Jose RissoNo ratings yet
- 2222Document3 pages2222ArunNo ratings yet
- 2222Document3 pages2222ArunNo ratings yet
- Velammal Institute of Technology Panchetti, Chennai - 601 204 Cycle Test-1Document1 pageVelammal Institute of Technology Panchetti, Chennai - 601 204 Cycle Test-1muru0105No ratings yet
- Austempering of Bearing Steel For Improved Mechanical PropertiesDocument4 pagesAustempering of Bearing Steel For Improved Mechanical PropertiesAhuti ChatterjeeNo ratings yet
- 금속재료 중간고사 기출문제 (2006-2016)Document10 pages금속재료 중간고사 기출문제 (2006-2016)Li Ken LokNo ratings yet
- Heat Transfer in Polymer Composite Materials: Forming ProcessesFrom EverandHeat Transfer in Polymer Composite Materials: Forming ProcessesNicolas BoyardNo ratings yet