Professional Documents
Culture Documents
Frantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case Report
Frantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case Report
Uploaded by
costachemfOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Frantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case Report
Frantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case Report
Uploaded by
costachemfCopyright:
Available Formats
JOP. J Pancreas (Online) 2009 Mar 9; 10(2):209-211.
CASE REPORT
Frantzs Tumor (Solid Pseudopapillary Tumor) of the Pancreas. A Case Report
Settar Bostanoglu, Emrah Otan, Saadet Akturan, Enver Okan Hamamci, Akin Bostanoglu, Aysun Gokce, Levent Albayrak Numune Training and Research Hospital. Ankara, Turkey
ABSTRACT Context A solid pseudopapillary tumor of the pancreas is a rare neoplasm which, for the most part, affects young women and has a relatively favorable prognosis with a low malignant potential. These tumors usually have unclear clinical features and may form very large masses before being diagnosed. Case report We report the case of a 29-year-old woman who underwent complete resection of the tumor using a distal pancreatectomy and splenectomy procedure. The patient is being followed-up and in good condition. A review of the relevant literature is also presented. Conclusions A solid pseudopapillary tumor of the pancreas is a rare condition with a low potential for malignancy and favorable prognosis; surgical resection is generally curative.
INTRODUCTION A solid pseudopapillary tumor of the pancreas is an rare neoplasm accounting for less than 2% of exocrine pancreatic neoplasms [1, 2]. This rare entity was first described by the author Dr. Frantz in 1959, and was called papillary tumor of the pancreas, benign or malignant [1, 3]. Until it was defined by the World Health Organization (WHO) in 1996 as solid pseudopapillary tumors of the pancreas, this tumor was described by using various names including solid cystic tumor, papillary cystic tumor, papillary epithelial neoplasia, solid and papillary epithelial neoplasia, papillary epithelial tumor, Frantzs tumor, solid and papillary tumor, solid-cysticpapillary epithelial neoplasm, benign or malignant papillary tumor of the pancreas and adenocarcinoma of the pancreas in childhood [4, 5]. These neoplasms with low malignant potential affect, for the most part, young women and tend to be treated with aggressive resection. After resection and follow-up, they generally have a relatively favorable prognosis [1]. We report our clinical experience with a solid pseudopapillary tumor and include a review of the current literature. CASE REPORT A previously healthy 29-year-old woman who presented with epigastric abdominal pain of 3 months
Received January 21st, 2009 - Accepted February 1st, 2009 Key words Carcinoma, Papillary; Pancreas; Pancreatic Neoplasms Correspondence Emrah Otan Emek Mah. 8.Cad. No:217/11, Cankaya, 06510, Ankara, Turkey Phone: +90-533.634.5856; Fax: +90-312.310.3460 E-mail: emrahotan@yahoo.de Document URL http://www.joplink.net/prev/200903/24.html
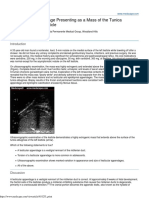
duration was admitted with an abdominal mass. She had had no previous history of hospitalization. Physical examination revealed mild epigastric tenderness and a palpable mass about 10 cm. in diameter, located in the abdomen extending from the upper left quadrant to the upper right quadrant. Routine laboratory test results including tumor marker levels (carcinoembryogenic antigen, alpha-fetoprotein, CA 19-9) were within normal limits. Abdominal ultrasonography demonstrated a heterogeneous semisolid mass with cystic components adjacent to the left lobe of the liver. On abdominal CT, a solid mass, 11 cm in diameter, involving the pancreatic corpus and tail was observed (Figure 1). An abdominal MRI scan defined the mass as originating from the pancreatic corpus and tail, 10 cm. in diameter. Arterial and venous Doppler ultrasonography examination showed the mass to have an intimate relationship with the splenic vein. At laparotomy, exploration revealed an encapsulated mass of 15 cm in diameter in the tail and body of the pancreas involving the splenic artery and vein. There was no evidence of intra-abdominal metastasis. Distal pancreatectomy and splenectomy were performed. A solid pseudopapillary tumor was diagnosed on pathologic examination of the specimen (Figure 2). The postoperative period was complicated by a localized cyst at the operation site which was diagnosed by a CT scan performed 11 days after surgery. The cyst was 10 cm in diameter and percutaneous aspiration was carried out using ultrasonographic guidance. Diagnostic laboratory tests showed high levels of amylase which was interpreted to be proof of pancreatic origin. Abdominal CT carried out 1 and 3 months after the surgery showed the diameter of the cyst to have decreased (2 cm); the
JOP. Journal of the Pancreas - http://www.joplink.net - Vol. 10, No. 2 - March 2009. [ISSN 1590-8577]
209
JOP. J Pancreas (Online) 2009 Mar 9; 10(2):209-211.
Figure 1. Computed tomographic scan. Encapsulated complex, solid and cystic mass, 11 cm in diameter, localized in corpus and tail of the pancreas.
physical examination findings and laboratory tests did not reveal any significant infectious process. The patient has been followed-up as an out-patient for 3 years and has remained healthy without any sign of local recurrence or distant metastasis. DISCUSSION Solid-pseudopapillary tumors are rare primary neoplasms of the pancreas with a low malignant potential which tend to occur primarily in young women [1, 2]. These neoplasms account for 5% of cystic pancreatic tumors and 1-2 % of exocrine pancreatic neoplasms [5]. There have been 718 cases reviewed in the English literature; female dominance has been found with a ratio of 10:1 and there is a mean age of 22 years [6]. The origin of solid pseudopapillary tumors still remains unclear. These neoplasms have been suggested to have a ductal epithelial, neuroendocrine, multipotent primordial cell, or even an extra-pancreatic genital ridge angle-related cell origin [5, 7]. The majority of the tumors are located in the pancreatic body and tail [5, 8, 9]. A combination of solid and cystic components with cellular degenerative changes alternating with pseudopapillary formation are frequently mentioned among the characteristic features observed with a light microscope which distinguish pseudopapillary tumors from other neoplasms of the pancreas [2, 10]. The solid portions of the tumor are composed of uniform and polygonal epithelioid cells with well-vascularized stroma and a discohesive arrangement [9, 10]. Specific findings for cellular differentiation in solid pseudopapillary tumor cells remain unclear and differential diagnosis requires immunohistochemical staining. Solid pseudopapillary tumor cells are usually absent when stains for endocrine markers are used (insulin, glucagon, somatostatin, serotonin, gastrin, vasoactive intestinal peptide, bombesine, chromogranin, lysosome and CD68) and are characteristically positive for vimentin, CD10, CD56, and alpha-1-antitrypsin [9, 10]. These cells may also reveal focal immunoreactivity for cytokeratin, neuron-specific enolase and synaptophysin, demonstrate abnormal nuclear localization of beta-catenin, present progesterone receptors and may express galectin-3, all of which are useful in differentiating them from endocrine pancreatic tumor cells [10, 11].
Most of the patients present with unclear clinical features including abdominal discomfort, mild abdominal pain, increased abdominal girth, poor appetite and nausea which are related to tumor compression on the adjacent organs [2, 6]. When a palpable mass is presented, the average size of the tumor becomes quite remarkable (8-10 cm) [2, 7]. The majority of the small tumors are occasionally diagnosed during complementary imaging studies such as ultrasound or CT scan of the abdomen [8, 12]. Solid pseudopapillary tumors have not been associated with specific clinical laboratory test findings including serum tumor markers [8, 9]. The radiologic features of the tumor present similarities in most cases. Although CT scanning is the most frequent technique in diagnosing a solid pseudopapillary tumor, sonographic examination and magnetic resonance imaging (MRI) also define these hypervascular, well-encapsulated round mass with mixed cystic and solid components [8, 12]. Echoendosonography may provide fine-needle puncture biopsy with an option of preoperative pathologic diagnosis of the tumor [12]. Despite the locally aggressive features, the tumor has a low-grade malignant potential and tends to have a favorable prognosis, even in the presence of metastatic disease [1, 7, 9]. Metastases with an incidence of 15%,
Figure 2. Tumor tissue presenting a notable encapsulation by the pancreatic parenchyma under low power (outer square, H&E, x4). Solid pseudopapillary tumor tissue presenting characteristic pseudopapillary architecture at a higher magnification (inner square, H&E, x100).
JOP. Journal of the Pancreas - http://www.joplink.net - Vol. 10, No. 2 - March 2009. [ISSN 1590-8577]
210
JOP. J Pancreas (Online) 2009 Mar 9; 10(2):209-211.
most of which are hepatic, and local recurrence have rarely been reported in the long term follow-up of these patients [1, 7, 8]. Most of the metastatic patients are male and these lesions are frequently found at the time of diagnosis [7, 8]. Lymph node involvement is a rare condition which has been reported in 4 cases to date [2, 10]. Overall 5-year survival is as high as 97% in patients undergoing surgical resection [2, 10]. A number of cases having a more aggressive but shorter clinical course leading to death have been reported in which the mean age of the patients is older and metastatic disease to the liver or peritoneum was diagnosed at the time of initial presentation [9, 10]. The mean survival rate of these patients was 90 months [10]. Surgery is the treatment of choice even in the case of distant hepatic metastasis or local recurrence which are not contraindications for surgical therapy [1, 6, 13, 14, 15]. There is limited experience regarding chemotherapy and radiotherapy with or without the presence of metastatic disease. Some experimental regimes have been used including 5-fluorouracil, doxorubicin, streptozocin, cisplatin, topotecan, iphosphamide, and etoposide without a significant clinical response [2, 12]. A favorable response to radiotherapy in locally advanced unresectable disease has been reported [1, 2]. In conclusion, a solid pseudopapillary tumor of the pancreas is a rare condition with a low potential for malignancy. These tumors usually affect women in the second or third decades of life. The prognosis is favorable even in the presence of distant metastasis. Although surgical resection is generally curative, a close follow-up is advised in order to diagnose a possible local recurrence or distant metastasis and choose the proper therapeutic option for the patient. Conflict of interest The authors have no potential conflicts of interest
References 1. Zinner MJ, Shurbaji MS, Cameron JL. Solid and papillary epithelial neoplasms of the pancreas. Surgery 1990; 108:475-80. [PMID 2396191]
2. Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solidpseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol 2002; 9:35-40. [PMID 11833495] 3. Frantz VK. Papillary tumors of the pancreas: Benign or malignant ? Tumors of the pancreas. In: Atlas of Tumor Pathology, Section 7, Fascicles 27 and 28.Washington, DC, USA: Armed Forces Institute of Pathology, 1959:32-3. 4. Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumors of the exocrine pancreas. In: World Health Organization International Histological Classification of Tumours 2nd ed Berlin, Heidelberg, New York: Springer, 1996: 8452/1. [ISBN 3-540-60280-1] 5. Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. J Pancreas (Online) 2006; 7:131-6. [PMID 16407635] 6. Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol 2006; 12:3180-5. [PMID 16718837] 7. Eder F, Schulz HU, Rcken C, Lippert H. Solid-pseudopapillary tumor of the pancreatic tail. World J Gastroenterol 2005; 11: 4117-9. [PMID 15996043] 8. Salvia R, Bassi C, Festa L, Falconi M, Crippa S, Butturini G, et al. Clinical and biological behavior of pancreatic solid pseudopapillary tumors: report on 31 consecutive patients. J Surg Oncol 2007; 95:304-10. [PMID 17326131] 9. Yoon DY, Hines OJ, Bilchik AJ, Lewin K, Cortina G, Reber HA. Solid and papillary epithelial neoplasms of the pancreas: aggressive resection for cure. Am Surg 2001; 67:1195-9. [PMID 11768829] 10. Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas. Am J Surg Pathol 2005; 29:512-9. [PMID 15767807] 11. Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, Rahier J, Sempoux C. Solid and pseudopapillary tumor of the pancreas-review and new insights into pathogenesis. Am J Surg Pathol 2006; 30:1243-9. [PMID 17001154] 12. Frago R, Fabregat J, Jorba R, Garca-Borobia F, Altet J, Serrano MT, Valls C. Solid pseudopapillary tumors of the pancreas: diagnosis and curative treatment. Rev Esp Enferm Dig 2006; 98:80916. [PMID 17198473] 13. Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg 2006; 93:733-7. [PMID 16609955] 14. Gedaly R, Toledano A, Millan G, Essenfeld H, Zambrano VJ. Treatment of liver metastases from a solid pseudopapillary tumor of the pancreas. J Hepatobiliary Pancreat Surg 2006; 13:587-90. [PMID 17139438] 15. Horisawa M, Niinomi N, Sato T, Yokoi S, Oda K, Ichikawa M, Hayakawa S. Frantz's tumor (solid and cystic tumor of the pancreas) with liver metastasis: successful treatment and long-term follow-up. J Pediatr Surg 1995; 30:724-6. [PMID 7623239]
JOP. Journal of the Pancreas - http://www.joplink.net - Vol. 10, No. 2 - March 2009. [ISSN 1590-8577]
211
You might also like
- Dog Acupressure Chart and Pressure PointsDocument5 pagesDog Acupressure Chart and Pressure PointskinezildiNo ratings yet
- YagciDocument7 pagesYagciPatricia BezneaNo ratings yet
- Recurrent Adenocarcinoma of Colon Presenting As Duo-Denal Metastasis With Partial Gastric Outlet Obstruction: A Case Report With Review of LiteratureDocument5 pagesRecurrent Adenocarcinoma of Colon Presenting As Duo-Denal Metastasis With Partial Gastric Outlet Obstruction: A Case Report With Review of LiteratureVika RatuNo ratings yet
- Carcinoid Tumor of The Appendix: A Case ReportDocument3 pagesCarcinoid Tumor of The Appendix: A Case ReportrywalkNo ratings yet
- File 1Document4 pagesFile 1Marsya Yulinesia LoppiesNo ratings yet
- SMJ 56 E134Document3 pagesSMJ 56 E134tyasNo ratings yet
- Nephroblastoma: Radiological and Pathological Diagnosis of A Case With Liver MetastasesDocument5 pagesNephroblastoma: Radiological and Pathological Diagnosis of A Case With Liver MetastasesfifahcantikNo ratings yet
- Primary Pure Squamous Cell Carcinoma of The Duodenum: A Case ReportDocument4 pagesPrimary Pure Squamous Cell Carcinoma of The Duodenum: A Case ReportGeorge CiorogarNo ratings yet
- Krukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseDocument3 pagesKrukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseradianrendratukanNo ratings yet
- Squamous Cell Carcinoma of The PancreasDocument2 pagesSquamous Cell Carcinoma of The Pancreaserick_khristianNo ratings yet
- 54 Raviswami EtalDocument3 pages54 Raviswami EtaleditorijmrhsNo ratings yet
- s40792 018 0489 1Document7 pagess40792 018 0489 1Laura PredescuNo ratings yet
- Synchronous Pancreatic Neuroendocrine Tumor and Pancreatic Cyst A Case ReportDocument4 pagesSynchronous Pancreatic Neuroendocrine Tumor and Pancreatic Cyst A Case ReportWorld Journal of Clinical SurgeryNo ratings yet
- Surgical Management of Solid Tumor-4674Document7 pagesSurgical Management of Solid Tumor-4674Theofhila AldelaNo ratings yet
- Pancreatic Head Mass: What Can Be Done ? Diagnosis: UltrasonographyDocument4 pagesPancreatic Head Mass: What Can Be Done ? Diagnosis: Ultrasonographynarsing59No ratings yet
- A Rare Case of The Urinary Bladder: Small Cell CarcinomaDocument3 pagesA Rare Case of The Urinary Bladder: Small Cell CarcinomaMuhammad MaulanaNo ratings yet
- The Histomorphological Study of Prostate LesionsDocument5 pagesThe Histomorphological Study of Prostate LesionsIOSRjournalNo ratings yet
- Pancreatic Head Mass: What Can Be Done ? Diagnosis: UltrasonographyDocument4 pagesPancreatic Head Mass: What Can Be Done ? Diagnosis: UltrasonographyMolla WariNo ratings yet
- English Task Gynecology System: Case ReportDocument4 pagesEnglish Task Gynecology System: Case ReportCecepNo ratings yet
- Pertanyaan Referat CA SigmoidDocument9 pagesPertanyaan Referat CA SigmoidBelladinaMMNo ratings yet
- Ardengh Et Al - 2013 - Accuracy of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in TheDocument7 pagesArdengh Et Al - 2013 - Accuracy of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in TheMaria ChenNo ratings yet
- International Journal of Pharmaceutical Science Invention (IJPSI)Document3 pagesInternational Journal of Pharmaceutical Science Invention (IJPSI)inventionjournalsNo ratings yet
- Mucoepidermoidcarcinoma of The Pancreas: A Case ReportDocument3 pagesMucoepidermoidcarcinoma of The Pancreas: A Case ReportIJAR JOURNALNo ratings yet
- 40 Year ReviewDocument8 pages40 Year Reviewali aydemirNo ratings yet
- BAB I PancreatoblastomaDocument5 pagesBAB I PancreatoblastomaHana YunikoNo ratings yet
- G. Yagci, S. Cetiner, M. Dede, O. GunhanDocument3 pagesG. Yagci, S. Cetiner, M. Dede, O. GunhanAnonymous pNIzA5No ratings yet
- JurnalDocument13 pagesJurnalAnonymous OSTAJzYWDDNo ratings yet
- Small Intestine TumorsDocument8 pagesSmall Intestine TumorspkotaphcNo ratings yet
- Low Peh Hueh International Medical University, MalaysiaDocument19 pagesLow Peh Hueh International Medical University, MalaysiaChaitanya Venkata SattiNo ratings yet
- Primary Desmoplastic Small-Round-Cell Tumor of The Ovary: Casereports Open AccessDocument4 pagesPrimary Desmoplastic Small-Round-Cell Tumor of The Ovary: Casereports Open AccessNehemias MD GuevaraNo ratings yet
- Malignant Transformation in An Ovarian Mature Cystic Teratoma: A Case ReportDocument4 pagesMalignant Transformation in An Ovarian Mature Cystic Teratoma: A Case ReportnajmulNo ratings yet
- Aghiz 3Document12 pagesAghiz 3nandaaa aprilNo ratings yet
- Fibrolamellar Variant of Hepatocellular Carcinoma in A Young FemaleDocument3 pagesFibrolamellar Variant of Hepatocellular Carcinoma in A Young FemaleIKIKIKIKIKI999999No ratings yet
- Wjarr 2024 0960Document4 pagesWjarr 2024 0960mcvallespinNo ratings yet
- Rare Peritoneal Tumour Presenting As Uterine Fibroid: Janu Mangala Kanthi, Sarala Sreedhar, Indu R. NairDocument3 pagesRare Peritoneal Tumour Presenting As Uterine Fibroid: Janu Mangala Kanthi, Sarala Sreedhar, Indu R. NairRezki WidiansyahNo ratings yet
- A Rare Germ-Cell Tumor Site: Vaginal Endodermal Sinus Tumor: CasereportDocument3 pagesA Rare Germ-Cell Tumor Site: Vaginal Endodermal Sinus Tumor: CasereportmonicamoniccNo ratings yet
- Rupture of Urinary Bladder: A Case Report and Review of LiteratureDocument3 pagesRupture of Urinary Bladder: A Case Report and Review of LiteratureAmanda Samurti PertiwiNo ratings yet
- 10-Case Report PDFDocument3 pages10-Case Report PDFDrAnamika SinghNo ratings yet
- Ijgo 12614Document20 pagesIjgo 12614Marco Julcamoro AsencioNo ratings yet
- Massa OavriumDocument3 pagesMassa OavriumAde Gustina SiahaanNo ratings yet
- Ovarian Cancer With The Metastatic Deposit in The Cervix As Rare Case: A Case Report and Literature ReviewDocument5 pagesOvarian Cancer With The Metastatic Deposit in The Cervix As Rare Case: A Case Report and Literature Reviewfaika oesmaniaNo ratings yet
- International Journal of Surgery Case Reports: Adenocarcinoma in An Ano-Vaginal Fistula in Crohn's DiseaseDocument4 pagesInternational Journal of Surgery Case Reports: Adenocarcinoma in An Ano-Vaginal Fistula in Crohn's DiseaseTegoeh RizkiNo ratings yet
- Near-Infrared Raman Spectroscopy For TheDocument12 pagesNear-Infrared Raman Spectroscopy For TheAlberto Carlos Espinosa GaravitoNo ratings yet
- Current Diagnosis and Management of Retroperitoneal SarcomaDocument11 pagesCurrent Diagnosis and Management of Retroperitoneal SarcomaMaximiliano TorresNo ratings yet
- Ovarian Cysts and Tumors in Infancy and Childhood: Riginal RticleDocument4 pagesOvarian Cysts and Tumors in Infancy and Childhood: Riginal RticleIwan Budianto HadiNo ratings yet
- Adnexal Masses in Pediatric and Adolescent Females: A Review of The LiteratureDocument8 pagesAdnexal Masses in Pediatric and Adolescent Females: A Review of The LiteratureAlejandro GuzmanNo ratings yet
- Metastatic Spread of Mucinous Cystadenocarcinoma of The Ovaries Into Abdominal WallDocument3 pagesMetastatic Spread of Mucinous Cystadenocarcinoma of The Ovaries Into Abdominal WallIsta Fatimah Kurnia RahmiNo ratings yet
- TangDocument8 pagesTangPatricia BezneaNo ratings yet
- A Rare Case of Colon Cancer in A Young Patient: A Case Report and Literature ReviewDocument5 pagesA Rare Case of Colon Cancer in A Young Patient: A Case Report and Literature ReviewIJAR JOURNALNo ratings yet
- Mucinous TumorsDocument9 pagesMucinous TumorsBadiu ElenaNo ratings yet
- Urethral Metastasis From A Sigmoid Colon Carcinoma: A Quite Rare Case Report and Review of The LiteratureDocument5 pagesUrethral Metastasis From A Sigmoid Colon Carcinoma: A Quite Rare Case Report and Review of The LiteratureIdaamsiyatiNo ratings yet
- Current Diagnosis and Management of Ovarian Cysts: Review ArticlesDocument4 pagesCurrent Diagnosis and Management of Ovarian Cysts: Review ArticlesFelNo ratings yet
- Pure Laparoscopic Management of A Giant Ovarian Cyst in An AdolescentDocument3 pagesPure Laparoscopic Management of A Giant Ovarian Cyst in An AdolescentValian IndrianyNo ratings yet
- 1 s2.0 S2214442015000388 MainDocument3 pages1 s2.0 S2214442015000388 MainDewa Made Sucipta PutraNo ratings yet
- Nej Mo A 0806347Document7 pagesNej Mo A 0806347Vhy AnnisaNo ratings yet
- Akiyama Case ReportDocument3 pagesAkiyama Case ReportApmc SchwartzNo ratings yet
- Multiple Bilateral Choroidal Metastasis From Anal MelanomaDocument2 pagesMultiple Bilateral Choroidal Metastasis From Anal MelanomaMoazzam FarooqiNo ratings yet
- Colon Adenocarcinoma With Metastasis To The GingivaDocument3 pagesColon Adenocarcinoma With Metastasis To The GingivaSafira T LNo ratings yet
- An In-Depth Look at Krukenberg TumorDocument6 pagesAn In-Depth Look at Krukenberg Tumor垂直马克No ratings yet
- Upper Tract Urothelial CarcinomaFrom EverandUpper Tract Urothelial CarcinomaShahrokh F. ShariatNo ratings yet
- Tumores Apendiculares: Salazar Velasco Jose Abraham R1Cg Departamento de Cirugía General Hospital Regional VillahermosaDocument22 pagesTumores Apendiculares: Salazar Velasco Jose Abraham R1Cg Departamento de Cirugía General Hospital Regional VillahermosaJose VelascoNo ratings yet
- Acute PancreatitisDocument46 pagesAcute PancreatitisLew NianNo ratings yet
- Testicular AppendagesDocument4 pagesTesticular AppendagesJohn stamosNo ratings yet
- GMC 2 Product Write-UpDocument15 pagesGMC 2 Product Write-UpVerodoxNo ratings yet
- Examining A Developmental Approach To Childhood ObesityDocument171 pagesExamining A Developmental Approach To Childhood ObesityAnonymous 8b1lZnQNo ratings yet
- PLEURA Efusion KuliahLDocument46 pagesPLEURA Efusion KuliahLLuna LitamiNo ratings yet
- Meta 2.1 Ocampo Libro (15-21)Document7 pagesMeta 2.1 Ocampo Libro (15-21)germanNo ratings yet
- Objective Structured Clinical Examination 2019Document148 pagesObjective Structured Clinical Examination 2019Nishtha KumarNo ratings yet
- About Nasopharyngeal Cancer: Overview and TypesDocument9 pagesAbout Nasopharyngeal Cancer: Overview and TypesJoelie-Ann NatanNo ratings yet
- Insignis Surgery 2 Gallbladder and Extrahepatic Biliary SystemDocument7 pagesInsignis Surgery 2 Gallbladder and Extrahepatic Biliary SystemPARADISE JanoNo ratings yet
- D. Plaseska-Karanfilska - Human Genetic Diseases-Intech (2011)Document298 pagesD. Plaseska-Karanfilska - Human Genetic Diseases-Intech (2011)Umair ZubairNo ratings yet
- OsteomyelitisDocument147 pagesOsteomyelitisAnkit Agur100% (1)
- Ena Respiratory EmergenciesDocument30 pagesEna Respiratory Emergencieseng78ineNo ratings yet
- Broncho PneumoniaDocument23 pagesBroncho Pneumoniaanon-84769398% (43)
- Breast Feeding (1-)Document52 pagesBreast Feeding (1-)Jho d great80% (5)
- Mata Tenang Visus Turun MendadakDocument75 pagesMata Tenang Visus Turun MendadakDianMuliasariNo ratings yet
- Urine Collection in CytologyDocument12 pagesUrine Collection in CytologyjunaidiabdhalimNo ratings yet
- IHC of Salivary DifferentiationDocument12 pagesIHC of Salivary DifferentiationMohamed ArafaNo ratings yet
- Guideline On Management of Dental Patients With Special Health Care NeedsDocument6 pagesGuideline On Management of Dental Patients With Special Health Care NeedsPriscila Belén Chuhuaicura SotoNo ratings yet
- Diagnosis of Spontaneous Canine Hyperadrenocorticism - 2012 ACVIM Consensus Statement (Small Animal)Document13 pagesDiagnosis of Spontaneous Canine Hyperadrenocorticism - 2012 ACVIM Consensus Statement (Small Animal)Maca Muñoz EscobedoNo ratings yet
- Russian Traditional Medicine and Its BenefitsDocument6 pagesRussian Traditional Medicine and Its BenefitsPirasan Traditional Medicine CenterNo ratings yet
- Pancreatic Adenocarcinoma: Albumin-Bound Paclitaxel/GemcitabineDocument1 pagePancreatic Adenocarcinoma: Albumin-Bound Paclitaxel/GemcitabineJaneNo ratings yet
- Simopoulos Omega3 Review 2004Document15 pagesSimopoulos Omega3 Review 2004Voicu ValeriuNo ratings yet
- Dr. Lamia El Wakeel, PhD. Lecturer of Clinical Pharmacy Ain Shams UniversityDocument19 pagesDr. Lamia El Wakeel, PhD. Lecturer of Clinical Pharmacy Ain Shams UniversitysamvetNo ratings yet
- Dynamic Partnerships in Philippine Health CareDocument2 pagesDynamic Partnerships in Philippine Health CareAnnilyn Velasco MiraballesNo ratings yet
- TalithaDocument4 pagesTalithaPharmex Kosova100% (1)
- Article 1Document4 pagesArticle 1Tashu SardaNo ratings yet
- UKALL14 ProtocolDocument10 pagesUKALL14 Protocolmesba HoqueNo ratings yet
- AM - ICD-10-AM GuideDocument26 pagesAM - ICD-10-AM GuidedrrskhanNo ratings yet