Professional Documents
Culture Documents
Organic Chemistry II Practice Exam #2 Answer Key
Organic Chemistry II Practice Exam #2 Answer Key
Uploaded by
Quang Hiep HaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry II Practice Exam #2 Answer Key
Organic Chemistry II Practice Exam #2 Answer Key
Uploaded by
Quang Hiep HaCopyright:
Available Formats
Organic Chemistry II: Practice Exam #2 Answer Key
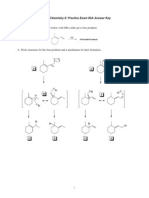
1. Propose structures for compounds A-E in the following reaction sequence. The molecular formula for E is provided.
2. Draw structures for the products of any five (5) of the following reactions. If you answer more than five, you will receive credit for the best five.
3. We have seen several examples in which protecting a ketone as a cyclic acetal allows synthesis of hydroxy-ketones such as 1 shown below. If 1 is subsequently heated in the presence of ptoluene sulfonic acid (an acid catalyst, H+), cyclic hemiacetal 2 is produced. Propose a mechanism for the conversion of 1 into 2.
Mechanism
4. Circle the compound in each pair that will have the lower pKa (i.e. will be the stronger acid).
5. Complete one of the two following syntheses. If you do both, you will receive credit for the better one. A. Starting from 1-bromopropane and any other reagents, synthesize 2-methyl-1-pentene.
The key thing to recognize is the presence of an alkene in the final product. This should make you think of a Wittig reaction. The first step in the retrosynthesis shows how the alkene product could come from the corresponding ketone. From there, another step back involves a change of oxidation state to an alcohol. The left half of the molecule could be provided by the starting material while the right half would come from a two carbon aldehyde. The full synthesis is shown below the retrosynthesis and shows how two different carbon-carbon bond-forming reactions are combined to make the final product.
B. Perform the synthesis shown below using any necessary reagents.
This problem looks simple: you could make the organolithium reagent, add propanal, then oxidize with PCC or H2CrO4.. However, as discussed in class, the organolithium reagent would be destroyed by taking a proton from the carboxylic acid group of another molecule. Since we do not know any protecting groups for carboxylic acids, you must first convert it into something that can be protected. For example, reduction to an alcohol allows you to add a THP protecting group. You can then set out to make the new carbon-carbon bond in the product. The retrosynthesis on the next page uses the following logic: the ketone in the final product likely came from an alcohol, since we know several ways to make a new C-C bond with an alcohol as the final product, but no methods (yet!) to make a new C-C bond with a ketone as the product. At the same time, the carboxylic acid can be retrod to a primary alcohol. Thinking forwards from this point, you would use H2CrO4.to oxidize the alcohols to the required ketone and carboxylic acid.
5
The next two steps show where the new C-C bond came from: an organolithium reagent (or Grignard) at the carbon where the bromine is attached in the starting material reacts with an aldehyde to yield the secondary alcohol. Introducing the THP protecting group at the appropriate time allows the organolithium reagent to be made and reacted with the propanal. The last retro step (and first forward step) shows conversion of the carboxylic acid into something that can be protected, an alcohol.
6. Solve one of the two following problems. If you do both, I will count the one on which you do better. A. Compound 1 is converted into compound 2 by reaction with chromic acid (H2CrO4). Based on the spectral data provided below, propose structures for 1 and 2. 1 (C5H8O2): IR (1710 cm-1), 1H-NMR 9.8 (s, 1H); 2.4 (t, 2H); 2.3 (t, 2H); 2.0 (s, 3H) 2 (C5H8O3)
There are two unsaturations in both compounds. NMR information for 1 gives an aldehyde (CHO), two CH2 groups and one CH3. This leaves CO from the formula, meaning that a ketone is likely present (also indicated by the IR). This is also consistent with the chemical shifts of the methyl and methylene groups. The only way to combine the five fragments to give a structure with the proper splitting is shown above. Chemical information (oxidation with chromic acid) indicates that the aldehyde is converted into a carboxylic acid. This matches the formula. B. Compound 3 has 1H-NMR spectral data given below. Based on this information, propose a structure for 3 and also write the structure of the product (4) that would form upon reaction of 3 with CH3MgBr followed by HCl/H2O. 3 (C9H18O2): IR (1710 cm-1); 1H-NMR 9H) 4.6 (s, 2H); 3.5 (s, 3H); 2.2 (t, 2H); 1.8 (t, 2H); 1.4 (s,
There is one unsaturation in 3 and the IR data indicates that this is a carbonyl. There is no aldehyde or carboxylic acid proton in the NMR spectrum, so this is likely a ketone. However, there are two oxygens in the molecule, so the second oxygen is either present as an alcohol or an ether. There is no signal in the NMR that integrates for one proton, so it cannot be an alcohol, so the second oxygen probably has two carbons bonded to it. The NMR data indicate the presence of a tbutyl group (s, 9H), two adjacent CH2 groups (two t, 2H), and isolated CH2 (s, 2H) and CH3 (s, 3H) groups. The chemical shifts for these last two groups are downfield, so it is likely that these are adjacent to the oxygen. The structure shown for 3 matches the NMR data.
You might also like
- Experiments in Physical Chemistry: Second Revised and Enlarged EditionFrom EverandExperiments in Physical Chemistry: Second Revised and Enlarged EditionNo ratings yet
- Organic Chemistry II Practice Exam #3A Answer KeyDocument8 pagesOrganic Chemistry II Practice Exam #3A Answer Keyhiep237No ratings yet
- Retrosynthetic Analysis PDFDocument6 pagesRetrosynthetic Analysis PDFHelmi FauziNo ratings yet
- CHM 2210 Practice Exam 1Document12 pagesCHM 2210 Practice Exam 1Shaima MossamatNo ratings yet
- Oxidation and Reduction Reactions in Organic ChemistryDocument9 pagesOxidation and Reduction Reactions in Organic ChemistryTarun Lfc Gerrard100% (1)
- Lab C-Methyl OrangeDocument4 pagesLab C-Methyl Orangetopikamew100% (1)
- Organic Chemistry 231 Final ExamDocument19 pagesOrganic Chemistry 231 Final ExamAlex Rose100% (1)
- Spectroscopic Solutions of StructureDocument21 pagesSpectroscopic Solutions of StructureKassimNo ratings yet
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiNo ratings yet
- Retrosynthesis SolutionsDocument7 pagesRetrosynthesis SolutionsScott Hendricks100% (1)
- Adv RetrosynthesisDocument29 pagesAdv RetrosynthesisSurya Dewi Wahyuningrum100% (1)
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- Retrosynthesis Practice Problems: O O OR O N O O BR O O OR 1)Document10 pagesRetrosynthesis Practice Problems: O O OR O N O O BR O O OR 1)butko88No ratings yet
- RetrosynthesisDocument20 pagesRetrosynthesistamilsam19860% (1)
- Reaction SummaryDocument5 pagesReaction SummaryShafaqatRahmanNo ratings yet
- Chemical KineticsDocument64 pagesChemical Kineticsnitiyan104552No ratings yet
- Organic Chemistry 2 Practice Exam 1Document15 pagesOrganic Chemistry 2 Practice Exam 1KaybidoNo ratings yet
- Disconnection in Organic SynthesisDocument31 pagesDisconnection in Organic SynthesisSwami Prabhu100% (2)
- Synthesis Review - Undergraduate Organic Synthesis GuideDocument19 pagesSynthesis Review - Undergraduate Organic Synthesis GuidePhạm Thị Thùy NhiênNo ratings yet
- Organic Chemistry Midterm 1 Dir+eff++keyDocument1 pageOrganic Chemistry Midterm 1 Dir+eff++keyNorma Leticia RamosNo ratings yet
- Practical Physical Chemistry CourseDocument68 pagesPractical Physical Chemistry CourseMahmoud AbdAllahNo ratings yet
- Kineticsss Notes PDFDocument73 pagesKineticsss Notes PDFArun SharmaNo ratings yet
- SN1, SN2, E1, E2Document39 pagesSN1, SN2, E1, E2Dian AnggrainiNo ratings yet
- NMR Solving StrategyDocument2 pagesNMR Solving Strategysorrow Lemon100% (1)
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocument9 pagesOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaNo ratings yet
- Disconnection ApproachDocument5 pagesDisconnection ApproachPrashanthPatroNo ratings yet
- Assignment 4 Reactions of Aromatic Compounds AnswersDocument11 pagesAssignment 4 Reactions of Aromatic Compounds AnswersJonathan Yeung100% (1)
- CARBONYL CONDENSATION REACTIONS 2 (10 Mei 2013)Document34 pagesCARBONYL CONDENSATION REACTIONS 2 (10 Mei 2013)Mammy Nya AllyaNo ratings yet
- Addition Reactions and Their MechanismsDocument47 pagesAddition Reactions and Their MechanismsttinbddinNo ratings yet
- OrganicChemistryChapter5 PDFDocument19 pagesOrganicChemistryChapter5 PDFJuliet Tatiana CumbeNo ratings yet
- CH 8 Handouts (All)Document34 pagesCH 8 Handouts (All)Ryan MaNo ratings yet
- Adv Retrosynthesis PDFDocument29 pagesAdv Retrosynthesis PDFericaNo ratings yet
- 11.alkenes and Alkynesexercise PDFDocument68 pages11.alkenes and Alkynesexercise PDFMohammed Owais KhanNo ratings yet
- Thermodynamic Versus Kinetic Reaction ControlDocument15 pagesThermodynamic Versus Kinetic Reaction ControlUdasi Raqs Kerti HaiNo ratings yet
- Alkenes SeatworkDocument5 pagesAlkenes SeatworkJhefNo ratings yet
- Free Radical Substitution and Electrophilic AdditionDocument17 pagesFree Radical Substitution and Electrophilic Additionchicko33No ratings yet
- Module8 PDFDocument40 pagesModule8 PDFFaizan AhmadNo ratings yet
- Report NMRDocument13 pagesReport NMRsarahNo ratings yet
- Welcome To Chem 206: Fall Term, 2005, David A. EvansDocument22 pagesWelcome To Chem 206: Fall Term, 2005, David A. EvanseraborNo ratings yet
- (+) - Vinblastine (111022-TKGP) T. Fukuyama, S. Yokoshima: ActivityDocument3 pages(+) - Vinblastine (111022-TKGP) T. Fukuyama, S. Yokoshima: ActivityPercival GalahadNo ratings yet
- Enamines and YlidesDocument18 pagesEnamines and YlidesVijay Pradhan100% (1)
- Organic Chemistry IIDocument7 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- Mechanism and Structure in Organic Chemistry-NGP-Gould PDFDocument60 pagesMechanism and Structure in Organic Chemistry-NGP-Gould PDFAJIT CHAUDHARINo ratings yet
- Organic Exam Answer.Document11 pagesOrganic Exam Answer.S JNo ratings yet
- 1 BenzeneDocument41 pages1 Benzeneraj royelNo ratings yet
- Terp e NoidsDocument46 pagesTerp e NoidsSunitha Katta100% (1)
- CH CH CCH C CHDocument15 pagesCH CH CCH C CHVirgilio Ebajo Jr.No ratings yet
- Alkyl HalidesDocument75 pagesAlkyl HalidesVikas GargNo ratings yet
- QOI0809 AlkenesDocument30 pagesQOI0809 Alkenesmtucker17No ratings yet
- Exams Organic Chemistry MITDocument333 pagesExams Organic Chemistry MITn2h_spNo ratings yet
- Reaction of Alkenes and Alkynes For StudentsDocument53 pagesReaction of Alkenes and Alkynes For StudentsGlen MangaliNo ratings yet
- 15 - Aldehyde and KetonesDocument66 pages15 - Aldehyde and KetonesIrfan Raza100% (1)
- Neighbouring Group ParticipationDocument15 pagesNeighbouring Group ParticipationAbdulMananNo ratings yet
- Sharpless Asymmetric EpoxidationDocument19 pagesSharpless Asymmetric Epoxidationjaanabhenchod100% (2)
- Exercises in Organic Synthesis Based on Synthetic DrugsFrom EverandExercises in Organic Synthesis Based on Synthetic DrugsNo ratings yet
- Hybrid Retrosynthesis: Organic Synthesis using Reaxys and SciFinderFrom EverandHybrid Retrosynthesis: Organic Synthesis using Reaxys and SciFinderNo ratings yet
- Reaction Kinetics: Reactions in SolutionFrom EverandReaction Kinetics: Reactions in SolutionRating: 3.5 out of 5 stars3.5/5 (4)
- Functional GroupDocument20 pagesFunctional GroupCatherine R. FelipeNo ratings yet
- Carboxylic Acid Questions-1Document6 pagesCarboxylic Acid Questions-1Jape GarridoNo ratings yet
- IUPAC NomenclatureDocument24 pagesIUPAC NomenclatureSougata DasNo ratings yet
- NCERT Solutions For Class 12 Chemistry Chapter 10 Haloalkanes and HaloarenesDocument39 pagesNCERT Solutions For Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenesabhik525No ratings yet
- DPP OcDocument196 pagesDPP Ocklrajshekhar9876No ratings yet
- Alkanes Y11Document15 pagesAlkanes Y11Iftitahur Rohmah -No ratings yet
- I0-Rs0.R-O-R'-O, R-Oh 4R-Oh: R0H-R Tagx-DoDocument2 pagesI0-Rs0.R-O-R'-O, R-Oh 4R-Oh: R0H-R Tagx-DoAvirup GhoshNo ratings yet
- Chemistry Pre-Board 2023Document3 pagesChemistry Pre-Board 2023Muhammad AhsanNo ratings yet
- Oxidation Via Iodine and Seo2Document14 pagesOxidation Via Iodine and Seo2Usman GhaniNo ratings yet
- Feasibility Study of Diphenyl Carbonate ProductionDocument4 pagesFeasibility Study of Diphenyl Carbonate ProductionIntratec SolutionsNo ratings yet
- Hidrocarbon REMED KIMIA RIZKY INDIDocument17 pagesHidrocarbon REMED KIMIA RIZKY INDIIndiNo ratings yet
- Chemical Test To Distinguish Between Pair of CompoundsDocument5 pagesChemical Test To Distinguish Between Pair of CompoundsIshika Singh100% (2)
- Aromatic It yDocument9 pagesAromatic It yBentenBentenNo ratings yet
- Aroma Chemistry - The Aroma of Frying BaconDocument1 pageAroma Chemistry - The Aroma of Frying BaconCarles Alcaide BlayaNo ratings yet
- NatSci 301 Instructional Learning Module 1Document7 pagesNatSci 301 Instructional Learning Module 1Grace VillanuevaNo ratings yet
- Chapter 22: Condensations and Alpha Substitutions of Carbonyl CompoundsDocument1 pageChapter 22: Condensations and Alpha Substitutions of Carbonyl CompoundsElizabeth Jean BaumeisterNo ratings yet
- Accord Mountain Fresh 8.5x11Document1 pageAccord Mountain Fresh 8.5x11KareemNo ratings yet
- 9.3 Alkenes and AlkynesDocument10 pages9.3 Alkenes and AlkynesMirjeta ZymeriNo ratings yet
- LipidsDocument3 pagesLipidsCamille GrandeNo ratings yet
- IR Spectroscopy Problem Set 4 Answer Key PDFDocument6 pagesIR Spectroscopy Problem Set 4 Answer Key PDFHuy Đặng AnhNo ratings yet
- FHSC1124 Report CoverDocument15 pagesFHSC1124 Report CovertravisthenNo ratings yet
- Organo Metals S 2018Document8 pagesOrgano Metals S 2018Muhammad Anas SoortyNo ratings yet
- Carbonyls, Carboxylic Acids and Chirality: Suffix - EneDocument20 pagesCarbonyls, Carboxylic Acids and Chirality: Suffix - EneAliya RahmanNo ratings yet
- Parker O-Ring Handbook (Modified)Document83 pagesParker O-Ring Handbook (Modified)precillaNo ratings yet
- Acquaintance With Covalent MoleculesDocument11 pagesAcquaintance With Covalent MoleculesAlfonsoNo ratings yet
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document21 pagesPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak Modi100% (1)
- LESSON PLAN HydrocarbonDocument7 pagesLESSON PLAN HydrocarbonNur LatifahNo ratings yet
- Nomenclature Alkanes Alkenes AlkynesDocument57 pagesNomenclature Alkanes Alkenes AlkynesWilhelm JulioNo ratings yet
- Assignment 4 Task 6Document9 pagesAssignment 4 Task 6peterNo ratings yet
- Exersice PDFDocument24 pagesExersice PDFharsh mishraNo ratings yet