Professional Documents
Culture Documents
RK
RK
Uploaded by
Kou UrakiCopyright:
Available Formats
You might also like
- A - Level Project Work Insights & ReflectionsDocument3 pagesA - Level Project Work Insights & ReflectionsKou Uraki0% (1)
- Core Practical 13a and 13b RevisionDocument15 pagesCore Practical 13a and 13b RevisionPriya KumarNo ratings yet
- Activation EnergyDocument9 pagesActivation Energyشكير قصطيNo ratings yet
- KongsbergDocument332 pagesKongsbergRoman100% (5)
- Scheme of Work Physics Year12 - 2019 2020Document8 pagesScheme of Work Physics Year12 - 2019 2020Tiras NgugiNo ratings yet
- Chemical Bonding - Practice Questions: Identify The Choice That Best Completes The Statement or Answers The QuestionDocument5 pagesChemical Bonding - Practice Questions: Identify The Choice That Best Completes The Statement or Answers The QuestionJemina R. B. Espedillon100% (1)
- 4.3 Reaction Rates and Reversible ReactionsDocument18 pages4.3 Reaction Rates and Reversible ReactionsVictor VC100% (6)
- CHEM 160 Formal Lab Report IDocument10 pagesCHEM 160 Formal Lab Report IDatoya BrownNo ratings yet
- A-Level Project Work Preliminary Investigations ExampleDocument5 pagesA-Level Project Work Preliminary Investigations ExampleKou Uraki43% (7)
- From Outer Space To You by Howard Menger Edited by Lucus Louize 2017 (Text Only Version)Document315 pagesFrom Outer Space To You by Howard Menger Edited by Lucus Louize 2017 (Text Only Version)Lib100% (4)
- Gabrielle Robinson - 601 Labs 2021Document13 pagesGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonNo ratings yet
- Experiment RORDocument1 pageExperiment RORfazdirNo ratings yet
- Nickezah ArthurDocument11 pagesNickezah ArthurchandanieeNo ratings yet
- 1.3 Rate of Reaction (1.2c)Document75 pages1.3 Rate of Reaction (1.2c)Sha Tasha Natasha0% (1)
- Chemistry SBA7 ReportDocument6 pagesChemistry SBA7 ReportSam ChanNo ratings yet
- Expt01 HCL and NaOH AnsDocument3 pagesExpt01 HCL and NaOH AnsaragpdNo ratings yet
- Chemistry Form 5 Chapter 1 - Rate of ReactionDocument63 pagesChemistry Form 5 Chapter 1 - Rate of ReactionSiti Nursyafiqah100% (7)
- 2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersDocument15 pages2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersonnoezNo ratings yet
- H2 Chemistry Prelims 2011 (Planning)Document12 pagesH2 Chemistry Prelims 2011 (Planning)iuhihzNo ratings yet
- Chemical KineticsDocument5 pagesChemical KineticsTimothy HandokoNo ratings yet
- Peka Tingkatan 5Document5 pagesPeka Tingkatan 5Sham Shinar0% (1)
- Exp. Rate of Reaction F.5Document6 pagesExp. Rate of Reaction F.5Gerard ಌஜಌ BoyzzNo ratings yet
- Experiment 2K3Document10 pagesExperiment 2K3Inkiru N. BernardNo ratings yet
- Rate NotesDocument16 pagesRate NotesMegan GohNo ratings yet
- Edexcel A2 Chemistry 4.3 - NotesDocument20 pagesEdexcel A2 Chemistry 4.3 - Notesjirwin588% (16)
- CHM3103 Lab Experiment 2Document15 pagesCHM3103 Lab Experiment 2husnaNo ratings yet
- 64edf0f4e41caDocument6 pages64edf0f4e41caDanzell JonathanNo ratings yet
- Chemistry SPM Potential Questions-Form5chap1 2Document15 pagesChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNo ratings yet
- Kinetics: 6.1 Rates of ReactionDocument20 pagesKinetics: 6.1 Rates of ReactionSeung Hee KimNo ratings yet
- Experiment 3 CHM476Document10 pagesExperiment 3 CHM476Hazwan Hamim100% (1)
- Gas Liquid AbsorptionDocument9 pagesGas Liquid AbsorptionShashwat OmarNo ratings yet
- Physical Exp1Document4 pagesPhysical Exp1shielasamvuraNo ratings yet
- Nyjc - 2007 Jc1 h2 Promo p2 - AnswerDocument12 pagesNyjc - 2007 Jc1 h2 Promo p2 - AnswerSudibyo GunawanNo ratings yet
- Rate of Reaction (Chapter 1)Document64 pagesRate of Reaction (Chapter 1)Timothy SimNo ratings yet
- Experiment 1 Ester SapnificationDocument14 pagesExperiment 1 Ester SapnificationTajTaj100% (1)
- Chem Lab - A Velocity Constant TitrationDocument6 pagesChem Lab - A Velocity Constant TitrationMiguel Ackah-Yensu50% (2)
- Tutorial 1 Rate of ReactionDocument2 pagesTutorial 1 Rate of ReactionchenanNo ratings yet
- Chemical Kinetics TutorialDocument2 pagesChemical Kinetics TutorialFormer TorrentNo ratings yet
- KineticsDocument6 pagesKineticsRain Y.No ratings yet
- 2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)Document6 pages2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)shakthee sivakumarNo ratings yet
- 9701 Nos Ps 20Document5 pages9701 Nos Ps 20lianchen251110No ratings yet
- Tutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Document7 pagesTutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Subesh ShanmugamNo ratings yet
- Rate of ReactionDocument9 pagesRate of ReactionShamshul Didarelly0% (1)
- N6lab 1Document9 pagesN6lab 1sachinkurhekarNo ratings yet
- Chemical Kinetics Methodology, RDRDocument7 pagesChemical Kinetics Methodology, RDRKhayzel MelanoNo ratings yet
- Sulfur Trioxide Concentrations PDFDocument12 pagesSulfur Trioxide Concentrations PDFRaraNo ratings yet
- Chapter 10 No 9Document8 pagesChapter 10 No 9Ain FzaNo ratings yet
- Physical Chemistry: SO H O H SODocument11 pagesPhysical Chemistry: SO H O H SOavmurugan87No ratings yet
- INSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Document6 pagesINSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Isabella Martins AndersenNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 10Document8 pagesRTS Chemistry SPM Question Bank Chapter 10Scorched ZenNo ratings yet
- 6 BEnergetics ZN Cu SO4Document6 pages6 BEnergetics ZN Cu SO4ROCKETMANNo ratings yet
- CH 1Document14 pagesCH 1Ketan RathodNo ratings yet
- Experiment oDocument5 pagesExperiment ogarzonhxc2No ratings yet
- Chapter 1 Rate of Reaction (Form 4 Chameistry)Document12 pagesChapter 1 Rate of Reaction (Form 4 Chameistry)siowling0922No ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- Example Planning Experiment Form 5Document14 pagesExample Planning Experiment Form 5Victoria PetrusNo ratings yet
- Unit 5 Chemical KineticsDocument37 pagesUnit 5 Chemical KineticsSanjay SharmaNo ratings yet
- Iodine Kinetics Clock ReactionDocument6 pagesIodine Kinetics Clock ReactionribotsNo ratings yet
- E3: Kinetics of The Hydrogen Peroxide/Iodide ReactionDocument5 pagesE3: Kinetics of The Hydrogen Peroxide/Iodide ReactionAlfian HadiwijayaNo ratings yet
- Experiment 3Document8 pagesExperiment 3Luxemberg NgNo ratings yet
- Rates of Reaction - Disappearing Cross LabDocument3 pagesRates of Reaction - Disappearing Cross Lab4L Anisha SieudassNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- 9647 Challenging QuestionsDocument1 page9647 Challenging QuestionsKou UrakiNo ratings yet
- A-Level General Paper AQ PracticeDocument3 pagesA-Level General Paper AQ PracticeKou Uraki0% (1)
- A-Level Project Work Evaluation of Materials ExampleDocument8 pagesA-Level Project Work Evaluation of Materials ExampleKou Uraki100% (5)
- Métodos de Diseño de Mezclas Asfálticas.Document151 pagesMétodos de Diseño de Mezclas Asfálticas.Fernando VaronNo ratings yet
- University of Guyana Faculty of Engineering and TechnologyDocument6 pagesUniversity of Guyana Faculty of Engineering and TechnologyBharat BalgobinNo ratings yet
- Design of Liquid-Liquid Extraction ColumnsDocument36 pagesDesign of Liquid-Liquid Extraction ColumnsJowel MercadoNo ratings yet
- AnsPhysics Ex-42 Hooke's LawDocument5 pagesAnsPhysics Ex-42 Hooke's LawLiuJiewChuanNo ratings yet
- Project Report On Erosion Wear of MaterialsDocument44 pagesProject Report On Erosion Wear of MaterialsNABIL HUSSAINNo ratings yet
- A Density Functional TheoryDocument8 pagesA Density Functional Theoryfebri_bontangNo ratings yet
- Ch01 PDFDocument33 pagesCh01 PDFphooolNo ratings yet
- Section-Active Structure SystemDocument21 pagesSection-Active Structure Systembowsar browsarNo ratings yet
- NABL 142 Policy On Calibration & TreasebilityDocument5 pagesNABL 142 Policy On Calibration & Treasebilitysudhasesh2000100% (2)
- Belt AnalysisDocument4 pagesBelt AnalysisMaheswaran MuthaiyanNo ratings yet
- 27.3 The Mechanical Energy Balance: Basic Principle II Second Class Dr. Arkan Jasim HadiDocument8 pages27.3 The Mechanical Energy Balance: Basic Principle II Second Class Dr. Arkan Jasim HadiSonu MishraNo ratings yet
- Pulse and Temperature Sensor (Presentation)Document14 pagesPulse and Temperature Sensor (Presentation)Ali HassanNo ratings yet
- Index: Abaqus, 6Document19 pagesIndex: Abaqus, 6Casao JonroeNo ratings yet
- Stresses Due To Fluid Pressure in Thin Cylinders (EngineeringDuniya - Com)Document25 pagesStresses Due To Fluid Pressure in Thin Cylinders (EngineeringDuniya - Com)BalvinderNo ratings yet
- Y 18 Hand Book - M.Tech - Y18 R (SE, CTM, GI) PDFDocument61 pagesY 18 Hand Book - M.Tech - Y18 R (SE, CTM, GI) PDFChandrakanth K JOeNo ratings yet
- Tubing Test Valve PsDocument0 pagesTubing Test Valve Pscamillo010No ratings yet
- Mod 6 Materials and Hardware: Exam PointsDocument16 pagesMod 6 Materials and Hardware: Exam PointsKetanRaoNo ratings yet
- Spacer Damper IssuesDocument5 pagesSpacer Damper IssuesTravis WoodNo ratings yet
- Failure Envelope of Artificially Cemented SandDocument5 pagesFailure Envelope of Artificially Cemented SandFelicioSantosNo ratings yet
- Integral Equations and Their ApplicationsDocument385 pagesIntegral Equations and Their Applicationsjmlanwar100% (9)
- Errata For Panofsky and Phillips PDFDocument2 pagesErrata For Panofsky and Phillips PDFplucht1No ratings yet
- Statics (Joint Method) PDFDocument69 pagesStatics (Joint Method) PDFSteven Dominic AballeNo ratings yet
- Hydrometer - Fluids Pressure Upthrust and Flotation - TutorvistaDocument6 pagesHydrometer - Fluids Pressure Upthrust and Flotation - TutorvistaRamaKrishnanGNo ratings yet
- Unit 8 States of MatterDocument9 pagesUnit 8 States of Mattersyaifulzubir1986100% (1)
- StanliDocument60 pagesStanligreg1212No ratings yet
- 167 Ginaca Pineapple Processing MachineDocument12 pages167 Ginaca Pineapple Processing MachineJunaid SayedNo ratings yet
RK
RK
Uploaded by
Kou UrakiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
RK
RK
Uploaded by
Kou UrakiCopyright:
Available Formats
INNOVA JUNIOR COLLEGE JC2 CHEMISTRY 2012 REACTION KINETICS PRACTICAL
TEACHERS COPY
NAME : __________________________ CG : ___________ DATE : ____________
Subject : Worksheet RK1:
Chemistry To determine the rate equation for the reaction: 2H+(aq) + S2O32 (aq) S (s) + SO2 (g) + H2O (l)
Duration : 1 hr 15 min
FA 1 is an aqueous solution containing 0.4 mol dm-3 aqueous sodium thiosulfate(VI). FA 2 is an aqueous solution containing 2.0 mol dm-3 aqueous hydrochloric acid. Hydrochloric acid reacts with sodium thiosulfate(VI) to produce yellow precipitate of sulfur. 2H+ (aq) + S2O32 (aq) S (s) + SO2 (g) + H2O (l) The rate equation for the reaction is: Rate = k[H+] [S2O32 ]
x
y
where x and y are the orders or reaction with respect to H+ and S2O32- respectively.
You are to determine the rate equation for the reaction between sodium thiosulfate (VI) and hydrochloric acid by determining the order of reaction with respect to sodium thiosulfate (VI) and hydrochloric acid.
You will determine the order of reaction for each reactant by varying the concentration of each species in turn, keeping the other reactant constant. The reaction can be observed using the precipitated sulfur which causes the solution to become opaque. The rate can be determined by measuring the time taken for a cross drawn on a piece of blank paper and placed under the reaction vessel to become obscured.
You are advised to read parts (a) to (i) before starting practical work.
Safety
1
You must wear safety eye protection throughout this experiment.
2.0 mol dm-3 of HCl is an irritant and corrosive. Wash all spillages with plenty of water.
SO2 is a choking gas which is toxic. Ensure good ventilation. Take care not to inhale the fumes. Asthmatics need to be particularly careful.
Sulfur is poisonous. Flush sink with lots of water during washing!
Experiments 1 to 4 (Varying the concentration of Na2S2O3) (a)Using a 100 cm3 measuring cylinder, measure 40 cm3 of FA 1. Pour the 40 cm3 of FA 1 into the 250 cm3 beaker and stand the beaker over a cross. Measure 30 cm3 of FA 2 into the other 100 cm3 measuring cylinder provided. Pour the 30 cm3 of FA 2 into the 250 cm3 beaker containing the FA 1 solution, simultaneously starting the stopwatch. Note the time taken, to the nearest second, for the cross to become obscured by the sulfur produced in the reaction. (Discard the reaction and wash the beaker immediately) Tabulate your results in the Table 1 below.
(b) Rinse the beaker and place 30 cm3 of FA 1 into the beaker from a measuring cylinder, together with 10 cm3 of distilled water from another measuring cylinder. 2
Measure a further 30 cm3 of FA 2 into the other measuring cylinder and pour this into the 250 cm3 beaker, noting the time taken for the cross to be obscured by the sulfur. Repeat the experiment with different volumes of FA1 and water (as shown in Table 1).
Experiments 5 to 7 (Varying the concentration of HCl) (c) Repeat the experiment using 30 cm3 of FA 1 and the volume of water as shown in the table. Add the volume of FA 2 as shown in the table and note the time taken for the cross to be obscured by the sulfur. Enter your result in Table 1 below.
Table 1 Experiment Volume of FA 2 / cm3 1 2 3 4 5 6 7 30 30 30 30 40 20 10 Volume of FA 1 / cm3 40 30 20 10 30 30 30 Volume of water / cm3 0 10 20 30 0 20 30 Time / s
1 / s-1 Time
8 11 17 39 10 12 14 0.125 0.0909 0.0588 0.0256 0.1 0.0833 0.0714 320 330 340 390 [2]
Rate
Vt
(d) Write an expression to show how the volume of S2O32- (V S 2O2 ) is related to the concentration 3 of S2O32- in the total volume (VT) of reaction mixture. Hence, conclude why it is important to keep the total volume of the reaction mixture constant. [2] Concentration of S2O32-
V S O 2
2 3
VT
[1]
It is important to keep the total volume of the reaction mixture constant to compare the concentrations of S2O32- easily for the various experiments [1].
(e) State and explain how increasing the volume of reactants will affect the rate of reaction and the time taken for sulfur to be formed. [2] Increasing the volume of reactants will increase the concentrations of the reactants. Thus the number of reactant particles increases. Frequency of effective collisions taking place in the reaction increases. [1] Since the rate of reaction is proportional to the frequency of effective collisions, the rate of reaction increases. The time taken for sulfur to be formed will decrease. [1] 3
Rate
1 where t = time taken for first appearance of sulfur t
(f) For Experiment 1 and 2, determine the order of reaction with respect to sodium thiosulphate and hydrochloric acid. [4] For Experiment 1, Vt is about constant PDO [1] OR When the volume of thiosulfate (FA1) is doubled, the rate is doubled. PDO [1] Hence Rate concentration Order of reaction with respect to sodium thiosulphate = 1 For Experiment 2, Rate = k ACE [1]
1 =k Time
Time is constant. PDO [1] OR When volume of acid (FA2) is doubled, rate remains unchanged. PDO [1] Order of reaction with respect to hydrochloric acid = 0 ACE[1]
(g) Suggest a graphical method to determine the order of reaction with respect to the two reactants. Explain the reason behind the graphs chosen. [2]
1 against volume. PDO [1] Time 1 This is because Rate and Volume of reactant Concentration of reactant. PDO [1] Time
The graph to plot will be (h) State the rate equation. Rate = k[H+] [S2O32 ]
0
1
[1] ACE [1]
(i) Suggest 2 possible sources of error in this experiment, and suggest improvements that could be made to overcome these errors. [4]
Error 1: It is difficult in deciding when to stop timing for the sulfur precipitate to form as the time at which the first precipitate of sulfur appears is quite ambiguous. In this context, we take that the same amount of sulfur is produced each time we first see the yellow colour of sulfur appearing. Improvement 1: A data logger with colourimeter could be used to determine more accurately the amount of sulfur produced through the use of a suitable probe to measure the colour intensity (which is proportional to the concentration of sulfur produced). That is when colour intensity 4
reaches a certain value (or when a certain amount of sulfur is produced), time will be noted accurately and when to stop timing. Error 2: Temperature may not be kept constant for the reaction to take place. This will affect the rate of reaction and cause the time taken for the precipitate of sulphur to appear to be inaccurate. Improvement 2: There are possible measures to reduce heat lost to surroundings such as perform the experiment in a wind-free room or carry out the experiment in a water bath with a controlled temperature. Error 3: Human reaction time in starting and stopping the stop watch will affect the time recorded for the sulfur to be formed. Improvement 3: Repeat the experiments to obtain average values.
Error 4: The time taken for the reaction is short which results in significant error due to human reaction time. This usually occurs if the amount of sulfur produced is small. Improvement 4: Use a slightly lower concentration of S2O32- as it will increase the reaction time. This will reduce the percentage error. However the time taken should not be too long as the experiment will not provide a good estimate to the initial rate.
(j) Suggest reason(s) why the order of reaction (x and y) with respect to H+ and S2O32 cannot be obtained from the stoichiometric equation directly. [1] The reaction is not an elementary reaction. Hence the rate equation is based on the ratedetermining step of the multiple-step mechanism.
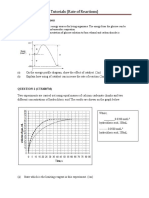
(k) Given the table below, estimate the time taken for the sulfur to obscure the cross. Hence explain how the rate of reaction is affected by the increase in temperature. [2]
Experiment 1 2
Fraction of particles
Volume of HCl / cm3 30 30
Volume of Na2S2O3 / cm3 40 40
Temp 30 40
Time/ s 8 <8
Low T The time taken for the sulfur to obscure the cross will be less than 8 seconds. [1]
Fraction of particles with E Ea at high T
Fraction of particles with E Ea at particles to increase. An increase in temperature will cause the average kinetic energyof the low T High T
There is an increase in the fraction of molecules with energy equal to or greater than the activation energy. Frequency of effective collisions taking place in the reaction increases. Since rate of reaction is proportional to the frequency of effective collisions, rate of reaction increases. [1]
5 0
Ea
Energy, E
You might also like
- A - Level Project Work Insights & ReflectionsDocument3 pagesA - Level Project Work Insights & ReflectionsKou Uraki0% (1)
- Core Practical 13a and 13b RevisionDocument15 pagesCore Practical 13a and 13b RevisionPriya KumarNo ratings yet
- Activation EnergyDocument9 pagesActivation Energyشكير قصطيNo ratings yet
- KongsbergDocument332 pagesKongsbergRoman100% (5)
- Scheme of Work Physics Year12 - 2019 2020Document8 pagesScheme of Work Physics Year12 - 2019 2020Tiras NgugiNo ratings yet
- Chemical Bonding - Practice Questions: Identify The Choice That Best Completes The Statement or Answers The QuestionDocument5 pagesChemical Bonding - Practice Questions: Identify The Choice That Best Completes The Statement or Answers The QuestionJemina R. B. Espedillon100% (1)
- 4.3 Reaction Rates and Reversible ReactionsDocument18 pages4.3 Reaction Rates and Reversible ReactionsVictor VC100% (6)
- CHEM 160 Formal Lab Report IDocument10 pagesCHEM 160 Formal Lab Report IDatoya BrownNo ratings yet
- A-Level Project Work Preliminary Investigations ExampleDocument5 pagesA-Level Project Work Preliminary Investigations ExampleKou Uraki43% (7)
- From Outer Space To You by Howard Menger Edited by Lucus Louize 2017 (Text Only Version)Document315 pagesFrom Outer Space To You by Howard Menger Edited by Lucus Louize 2017 (Text Only Version)Lib100% (4)
- Gabrielle Robinson - 601 Labs 2021Document13 pagesGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonNo ratings yet
- Experiment RORDocument1 pageExperiment RORfazdirNo ratings yet
- Nickezah ArthurDocument11 pagesNickezah ArthurchandanieeNo ratings yet
- 1.3 Rate of Reaction (1.2c)Document75 pages1.3 Rate of Reaction (1.2c)Sha Tasha Natasha0% (1)
- Chemistry SBA7 ReportDocument6 pagesChemistry SBA7 ReportSam ChanNo ratings yet
- Expt01 HCL and NaOH AnsDocument3 pagesExpt01 HCL and NaOH AnsaragpdNo ratings yet
- Chemistry Form 5 Chapter 1 - Rate of ReactionDocument63 pagesChemistry Form 5 Chapter 1 - Rate of ReactionSiti Nursyafiqah100% (7)
- 2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersDocument15 pages2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersonnoezNo ratings yet
- H2 Chemistry Prelims 2011 (Planning)Document12 pagesH2 Chemistry Prelims 2011 (Planning)iuhihzNo ratings yet
- Chemical KineticsDocument5 pagesChemical KineticsTimothy HandokoNo ratings yet
- Peka Tingkatan 5Document5 pagesPeka Tingkatan 5Sham Shinar0% (1)
- Exp. Rate of Reaction F.5Document6 pagesExp. Rate of Reaction F.5Gerard ಌஜಌ BoyzzNo ratings yet
- Experiment 2K3Document10 pagesExperiment 2K3Inkiru N. BernardNo ratings yet
- Rate NotesDocument16 pagesRate NotesMegan GohNo ratings yet
- Edexcel A2 Chemistry 4.3 - NotesDocument20 pagesEdexcel A2 Chemistry 4.3 - Notesjirwin588% (16)
- CHM3103 Lab Experiment 2Document15 pagesCHM3103 Lab Experiment 2husnaNo ratings yet
- 64edf0f4e41caDocument6 pages64edf0f4e41caDanzell JonathanNo ratings yet
- Chemistry SPM Potential Questions-Form5chap1 2Document15 pagesChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNo ratings yet
- Kinetics: 6.1 Rates of ReactionDocument20 pagesKinetics: 6.1 Rates of ReactionSeung Hee KimNo ratings yet
- Experiment 3 CHM476Document10 pagesExperiment 3 CHM476Hazwan Hamim100% (1)
- Gas Liquid AbsorptionDocument9 pagesGas Liquid AbsorptionShashwat OmarNo ratings yet
- Physical Exp1Document4 pagesPhysical Exp1shielasamvuraNo ratings yet
- Nyjc - 2007 Jc1 h2 Promo p2 - AnswerDocument12 pagesNyjc - 2007 Jc1 h2 Promo p2 - AnswerSudibyo GunawanNo ratings yet
- Rate of Reaction (Chapter 1)Document64 pagesRate of Reaction (Chapter 1)Timothy SimNo ratings yet
- Experiment 1 Ester SapnificationDocument14 pagesExperiment 1 Ester SapnificationTajTaj100% (1)
- Chem Lab - A Velocity Constant TitrationDocument6 pagesChem Lab - A Velocity Constant TitrationMiguel Ackah-Yensu50% (2)
- Tutorial 1 Rate of ReactionDocument2 pagesTutorial 1 Rate of ReactionchenanNo ratings yet
- Chemical Kinetics TutorialDocument2 pagesChemical Kinetics TutorialFormer TorrentNo ratings yet
- KineticsDocument6 pagesKineticsRain Y.No ratings yet
- 2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)Document6 pages2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)shakthee sivakumarNo ratings yet
- 9701 Nos Ps 20Document5 pages9701 Nos Ps 20lianchen251110No ratings yet
- Tutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Document7 pagesTutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Subesh ShanmugamNo ratings yet
- Rate of ReactionDocument9 pagesRate of ReactionShamshul Didarelly0% (1)
- N6lab 1Document9 pagesN6lab 1sachinkurhekarNo ratings yet
- Chemical Kinetics Methodology, RDRDocument7 pagesChemical Kinetics Methodology, RDRKhayzel MelanoNo ratings yet
- Sulfur Trioxide Concentrations PDFDocument12 pagesSulfur Trioxide Concentrations PDFRaraNo ratings yet
- Chapter 10 No 9Document8 pagesChapter 10 No 9Ain FzaNo ratings yet
- Physical Chemistry: SO H O H SODocument11 pagesPhysical Chemistry: SO H O H SOavmurugan87No ratings yet
- INSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Document6 pagesINSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Isabella Martins AndersenNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 10Document8 pagesRTS Chemistry SPM Question Bank Chapter 10Scorched ZenNo ratings yet
- 6 BEnergetics ZN Cu SO4Document6 pages6 BEnergetics ZN Cu SO4ROCKETMANNo ratings yet
- CH 1Document14 pagesCH 1Ketan RathodNo ratings yet
- Experiment oDocument5 pagesExperiment ogarzonhxc2No ratings yet
- Chapter 1 Rate of Reaction (Form 4 Chameistry)Document12 pagesChapter 1 Rate of Reaction (Form 4 Chameistry)siowling0922No ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- Example Planning Experiment Form 5Document14 pagesExample Planning Experiment Form 5Victoria PetrusNo ratings yet
- Unit 5 Chemical KineticsDocument37 pagesUnit 5 Chemical KineticsSanjay SharmaNo ratings yet
- Iodine Kinetics Clock ReactionDocument6 pagesIodine Kinetics Clock ReactionribotsNo ratings yet
- E3: Kinetics of The Hydrogen Peroxide/Iodide ReactionDocument5 pagesE3: Kinetics of The Hydrogen Peroxide/Iodide ReactionAlfian HadiwijayaNo ratings yet
- Experiment 3Document8 pagesExperiment 3Luxemberg NgNo ratings yet
- Rates of Reaction - Disappearing Cross LabDocument3 pagesRates of Reaction - Disappearing Cross Lab4L Anisha SieudassNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- 9647 Challenging QuestionsDocument1 page9647 Challenging QuestionsKou UrakiNo ratings yet
- A-Level General Paper AQ PracticeDocument3 pagesA-Level General Paper AQ PracticeKou Uraki0% (1)
- A-Level Project Work Evaluation of Materials ExampleDocument8 pagesA-Level Project Work Evaluation of Materials ExampleKou Uraki100% (5)
- Métodos de Diseño de Mezclas Asfálticas.Document151 pagesMétodos de Diseño de Mezclas Asfálticas.Fernando VaronNo ratings yet
- University of Guyana Faculty of Engineering and TechnologyDocument6 pagesUniversity of Guyana Faculty of Engineering and TechnologyBharat BalgobinNo ratings yet
- Design of Liquid-Liquid Extraction ColumnsDocument36 pagesDesign of Liquid-Liquid Extraction ColumnsJowel MercadoNo ratings yet
- AnsPhysics Ex-42 Hooke's LawDocument5 pagesAnsPhysics Ex-42 Hooke's LawLiuJiewChuanNo ratings yet
- Project Report On Erosion Wear of MaterialsDocument44 pagesProject Report On Erosion Wear of MaterialsNABIL HUSSAINNo ratings yet
- A Density Functional TheoryDocument8 pagesA Density Functional Theoryfebri_bontangNo ratings yet
- Ch01 PDFDocument33 pagesCh01 PDFphooolNo ratings yet
- Section-Active Structure SystemDocument21 pagesSection-Active Structure Systembowsar browsarNo ratings yet
- NABL 142 Policy On Calibration & TreasebilityDocument5 pagesNABL 142 Policy On Calibration & Treasebilitysudhasesh2000100% (2)
- Belt AnalysisDocument4 pagesBelt AnalysisMaheswaran MuthaiyanNo ratings yet
- 27.3 The Mechanical Energy Balance: Basic Principle II Second Class Dr. Arkan Jasim HadiDocument8 pages27.3 The Mechanical Energy Balance: Basic Principle II Second Class Dr. Arkan Jasim HadiSonu MishraNo ratings yet
- Pulse and Temperature Sensor (Presentation)Document14 pagesPulse and Temperature Sensor (Presentation)Ali HassanNo ratings yet
- Index: Abaqus, 6Document19 pagesIndex: Abaqus, 6Casao JonroeNo ratings yet
- Stresses Due To Fluid Pressure in Thin Cylinders (EngineeringDuniya - Com)Document25 pagesStresses Due To Fluid Pressure in Thin Cylinders (EngineeringDuniya - Com)BalvinderNo ratings yet
- Y 18 Hand Book - M.Tech - Y18 R (SE, CTM, GI) PDFDocument61 pagesY 18 Hand Book - M.Tech - Y18 R (SE, CTM, GI) PDFChandrakanth K JOeNo ratings yet
- Tubing Test Valve PsDocument0 pagesTubing Test Valve Pscamillo010No ratings yet
- Mod 6 Materials and Hardware: Exam PointsDocument16 pagesMod 6 Materials and Hardware: Exam PointsKetanRaoNo ratings yet
- Spacer Damper IssuesDocument5 pagesSpacer Damper IssuesTravis WoodNo ratings yet
- Failure Envelope of Artificially Cemented SandDocument5 pagesFailure Envelope of Artificially Cemented SandFelicioSantosNo ratings yet
- Integral Equations and Their ApplicationsDocument385 pagesIntegral Equations and Their Applicationsjmlanwar100% (9)
- Errata For Panofsky and Phillips PDFDocument2 pagesErrata For Panofsky and Phillips PDFplucht1No ratings yet
- Statics (Joint Method) PDFDocument69 pagesStatics (Joint Method) PDFSteven Dominic AballeNo ratings yet
- Hydrometer - Fluids Pressure Upthrust and Flotation - TutorvistaDocument6 pagesHydrometer - Fluids Pressure Upthrust and Flotation - TutorvistaRamaKrishnanGNo ratings yet
- Unit 8 States of MatterDocument9 pagesUnit 8 States of Mattersyaifulzubir1986100% (1)
- StanliDocument60 pagesStanligreg1212No ratings yet
- 167 Ginaca Pineapple Processing MachineDocument12 pages167 Ginaca Pineapple Processing MachineJunaid SayedNo ratings yet