Professional Documents
Culture Documents
Fall 2008 Quiz A Key
Fall 2008 Quiz A Key
Uploaded by
sarahabdulkareemCopyright:
Available Formats
You might also like
- TestFinal 350 v1 AnswersDocument8 pagesTestFinal 350 v1 AnswersJabe KoyNo ratings yet
- Stereochemistry Worksheet LabDocument3 pagesStereochemistry Worksheet LabDaniel McDermott0% (1)
- CMP Final Draft FuelDocument33 pagesCMP Final Draft Fuelapi-594648232No ratings yet
- Chapter 21 Further Aspects of EquilibriaDocument6 pagesChapter 21 Further Aspects of EquilibriaAndrea MelissaNo ratings yet
- CHEM1280 2012 13 Midterm Exam Solution PDFDocument5 pagesCHEM1280 2012 13 Midterm Exam Solution PDFLouisNo ratings yet
- 4b Evidence For Chemical Reactions LabDocument3 pages4b Evidence For Chemical Reactions Labapi-369690183100% (1)
- Chem1AA3 Lecture 1 PDFDocument110 pagesChem1AA3 Lecture 1 PDFbhavanjeetNo ratings yet
- Unit 8 EM MCQ Hydrocarbons 1991-2017Document24 pagesUnit 8 EM MCQ Hydrocarbons 1991-2017Imalka NanayakkaraNo ratings yet
- Chemistry Practice TestDocument2 pagesChemistry Practice Testyo mamaNo ratings yet
- Physics ReviewerDocument4 pagesPhysics ReviewermeriiNo ratings yet
- CH 2 Sec 1 Measurement-PowerpointDocument23 pagesCH 2 Sec 1 Measurement-Powerpointapi-294483847100% (2)
- MCQ Practice On (Chapter-3: Chemistry 1 Paper)Document4 pagesMCQ Practice On (Chapter-3: Chemistry 1 Paper)Mahin AzizNo ratings yet
- Exam 1 AnswersDocument9 pagesExam 1 AnswersArvic Lacson0% (1)
- Chemistry 2016 ExamsDocument20 pagesChemistry 2016 ExamsHoàng MinhNo ratings yet
- 11 Chemistry of Urine 1Document7 pages11 Chemistry of Urine 1Harold ApostolNo ratings yet
- Organic Chemistry TestDocument1 pageOrganic Chemistry Testron971No ratings yet
- 102 MSJC 13Document11 pages102 MSJC 13noelNo ratings yet
- Organic As Test P-2Document9 pagesOrganic As Test P-2zafarchem_iqbalNo ratings yet
- Flame TestsDocument4 pagesFlame TestsFelicia CharlesNo ratings yet
- Chem 132 2019 Tutorial QuestionsDocument3 pagesChem 132 2019 Tutorial QuestionsYusuf Zaynab100% (1)
- Inorganic Chemistry II (100 Items)Document11 pagesInorganic Chemistry II (100 Items)maria jeusa matiasNo ratings yet
- Mass Spectrometry (Multiple Choice) QPDocument12 pagesMass Spectrometry (Multiple Choice) QPYusuf AlamNo ratings yet
- Sample ProblemsDocument48 pagesSample Problemsapi-3856754No ratings yet
- Quiz 2 - Measurements and DensityDocument4 pagesQuiz 2 - Measurements and DensityCarolyn CampitaNo ratings yet
- 10 Lattice Energy - SDocument5 pages10 Lattice Energy - SisabelleNo ratings yet
- Unit-1 - AN OVERVIEW OF FOOD ChemistryDocument12 pagesUnit-1 - AN OVERVIEW OF FOOD ChemistryRadwan Ajo100% (1)
- PRACTICE MCQ HYDROCARBONS - 11ScADocument7 pagesPRACTICE MCQ HYDROCARBONS - 11ScAArda Rahmaini100% (1)
- Spectroscopy and ChromatographyDocument7 pagesSpectroscopy and ChromatographyPa GesNo ratings yet
- Organic Chemistry 2 Practice Exam 1Document15 pagesOrganic Chemistry 2 Practice Exam 1KaybidoNo ratings yet
- Worksheet 15 (CH 12, 13, 14)Document3 pagesWorksheet 15 (CH 12, 13, 14)Faheem ErshadNo ratings yet
- Chemistry 2 Answer KeyDocument8 pagesChemistry 2 Answer KeyMarielle BuesingNo ratings yet
- Atomic Structure: Examples of Multiple Choice QuestionsDocument4 pagesAtomic Structure: Examples of Multiple Choice Questionsngah lidwineNo ratings yet
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- Titrations in Analytical ChemistryDocument5 pagesTitrations in Analytical ChemistryBarronAliShapiNo ratings yet
- PLTL Ch. 16 AssignmentDocument6 pagesPLTL Ch. 16 AssignmentJules BrunoNo ratings yet
- Chemistry QuizDocument2 pagesChemistry Quizanon_572243106No ratings yet
- Carbenes and Nitrenes by IIT CampusDocument13 pagesCarbenes and Nitrenes by IIT CampusHemant soni100% (1)
- Chapter 3 - CALCULATIONS WITH CHEMICAL FORMULASDocument24 pagesChapter 3 - CALCULATIONS WITH CHEMICAL FORMULASSai RaghavaNo ratings yet
- 07 Introduction To Organic ChemistryDocument28 pages07 Introduction To Organic ChemistryM BNo ratings yet
- APCHEM Review Practice Test 1Document16 pagesAPCHEM Review Practice Test 1M. JosephNo ratings yet
- All Year Chemistry Up To 2018 PDFDocument37 pagesAll Year Chemistry Up To 2018 PDFAGAH LUCKYNo ratings yet
- 2013 As Chemistry Chapter 11 Review - GASESDocument11 pages2013 As Chemistry Chapter 11 Review - GASESbiancae697No ratings yet
- Stereo Chemistry QuestionsDocument19 pagesStereo Chemistry QuestionsfritzNo ratings yet
- Acid Base SolutionsDocument10 pagesAcid Base SolutionsCasey SangalliNo ratings yet
- ICSE Chemistry Board Paper19 PDFDocument9 pagesICSE Chemistry Board Paper19 PDFPrajakta DigheNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- CH 07Document96 pagesCH 07Jacquot AbendrothNo ratings yet
- MCQ On Periodic Classification of ElementsDocument2 pagesMCQ On Periodic Classification of ElementsNanda Rani SenNo ratings yet
- Hrushikesh Organic Group 5Document10 pagesHrushikesh Organic Group 5Sarita YadavNo ratings yet
- 2013 Organic Chemistry Exam First YearDocument7 pages2013 Organic Chemistry Exam First YearAnonymous oqlnO8eNo ratings yet
- Module8 PDFDocument40 pagesModule8 PDFFaizan AhmadNo ratings yet
- Alkenes and Alkynes II:: Addition ReactionsDocument102 pagesAlkenes and Alkynes II:: Addition Reactionsfingil20032003No ratings yet
- Chemical Reactions: John A. Schreifels Chemistry 211-Notes 1Document22 pagesChemical Reactions: John A. Schreifels Chemistry 211-Notes 1Hayan LeeNo ratings yet
- Applied Chemistry MCQsDocument10 pagesApplied Chemistry MCQsiangarvins100% (1)
- Class Test 1: Section A (Multiple-Choice Questions)Document10 pagesClass Test 1: Section A (Multiple-Choice Questions)Kgaugelo TraciaNo ratings yet
- Surface Chemistry MCQs - Questions - Paper 1Document7 pagesSurface Chemistry MCQs - Questions - Paper 1krishna prasad ghantaNo ratings yet
- 1.intro To Organic Chem-PracticeDocument2 pages1.intro To Organic Chem-PracticeZul Abror Bin Ya'akopNo ratings yet
- 19.2 Acid-Base Titration CurvesDocument9 pages19.2 Acid-Base Titration CurvesYuyun Sri IriantiNo ratings yet
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisFrom EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisRating: 4 out of 5 stars4/5 (2)
- Lab 5 Lab Plant Pigments 3-23-09Document4 pagesLab 5 Lab Plant Pigments 3-23-09Bayu PutraNo ratings yet
- Phloxine BDocument7 pagesPhloxine BDeepa DevanathanNo ratings yet
- 80010768Document11 pages80010768ArmanNo ratings yet
- Ethics of Student Final PDFDocument15 pagesEthics of Student Final PDFAmtul KafiNo ratings yet
- SkincareDocument84 pagesSkincareNikoletta AslanidiNo ratings yet
- Int Er Ene Rgy: Ie210 210/M/55/MHDocument2 pagesInt Er Ene Rgy: Ie210 210/M/55/MHRusnacNo ratings yet
- Military Engineer Services Notice Inviting Tender (Nit)Document14 pagesMilitary Engineer Services Notice Inviting Tender (Nit)jatanNo ratings yet
- Class 9 GrammarDocument32 pagesClass 9 GrammarPerajothi PalanirajaNo ratings yet
- Ilovepdf MergedDocument144 pagesIlovepdf MergedChandra PrakashNo ratings yet
- Component Identification of GearDocument6 pagesComponent Identification of GearWandiNo ratings yet
- Tos - TrendsDocument2 pagesTos - TrendsBernadette Falceso100% (1)
- Consilium Navigation Sal Broschyr PDFDocument8 pagesConsilium Navigation Sal Broschyr PDFSeamen 777No ratings yet
- MSDS Enercept - Ec1304aDocument11 pagesMSDS Enercept - Ec1304aKader Rahmani100% (1)
- Notification No. 477 (E) Dated 25th July, 1991 of The Department of Industrial Policy and Promotion, Ministry of IndustryDocument39 pagesNotification No. 477 (E) Dated 25th July, 1991 of The Department of Industrial Policy and Promotion, Ministry of IndustryHarish100% (3)
- Top Survival Tips - Kevin Reeve - OnPoint Tactical PDFDocument8 pagesTop Survival Tips - Kevin Reeve - OnPoint Tactical PDFBillLudley5100% (1)
- Keep 425Document12 pagesKeep 425Johan SukweenadhiNo ratings yet
- Threat Modeling Tool 2016 User GuideDocument59 pagesThreat Modeling Tool 2016 User GuideSenthilkumarmoorthyNo ratings yet
- Annotated BibliographyDocument2 pagesAnnotated Bibliographyapi-254427588No ratings yet
- Clarinet GroveDocument38 pagesClarinet GroveLaMusica IlMio:nomeNo ratings yet
- Numerical ModellingDocument119 pagesNumerical Modellingjanusz_1025No ratings yet
- Android Quiz App 22617 MADDocument19 pagesAndroid Quiz App 22617 MADSandip kotkarNo ratings yet
- Digital Storage Oscilloscopes: TDS210 TDS220 TDS224Document4 pagesDigital Storage Oscilloscopes: TDS210 TDS220 TDS224Jime SiepeNo ratings yet
- Airburst - Hendrick.r17 LRDocument2 pagesAirburst - Hendrick.r17 LRManuel Javier Rodríguez VNo ratings yet
- Literature Review On Hospital Information SystemsDocument8 pagesLiterature Review On Hospital Information Systemsc5mr3mxfNo ratings yet
- Newborn Studio Guide by Jessica G. PhotographyDocument13 pagesNewborn Studio Guide by Jessica G. PhotographyClau ppNo ratings yet
- Steven Johnsons SyndromeDocument22 pagesSteven Johnsons SyndromeRoselene Mae MarasiganNo ratings yet
- Case Shaping Future Business Leaders 9 June 2016Document7 pagesCase Shaping Future Business Leaders 9 June 2016Alka AggarwalNo ratings yet
- 2004 Waziristan Complex Chem Data Interptretaion SHAKIRULLAH & MOHAMMAD IHSAN AFRIDIDocument16 pages2004 Waziristan Complex Chem Data Interptretaion SHAKIRULLAH & MOHAMMAD IHSAN AFRIDInoor abdullahNo ratings yet
- Performance Appraisal at Jindal Brothers Pvt. LTD: A Dissertation Report ONDocument50 pagesPerformance Appraisal at Jindal Brothers Pvt. LTD: A Dissertation Report ONAvinash ThakurNo ratings yet
- Rehab-Plans-and-Exercises Hip-Arthroscopy Protocol-For-Physiotherapy-Following-SurgeryDocument2 pagesRehab-Plans-and-Exercises Hip-Arthroscopy Protocol-For-Physiotherapy-Following-SurgeryMellow Moon RecordsNo ratings yet
- Jerkins The FounderDocument15 pagesJerkins The FounderRifqi Khairul AnamNo ratings yet
Fall 2008 Quiz A Key
Fall 2008 Quiz A Key
Uploaded by
sarahabdulkareemOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fall 2008 Quiz A Key
Fall 2008 Quiz A Key
Uploaded by
sarahabdulkareemCopyright:
Available Formats
Chemistry 2311, Fall 2008
Student Name ______________________________ Quiz #1A (75 pts) 1.
Last Name of TA _______________
Waste Handling - Specifically describe how to handle each of the following waste materials generated during an experiment in lab. 8 pts (+ 2 each) a. a used chromatography column containing silica gel and solvent flush the column dry with air to remove all solvent. (The solvent would be place in organic hazardous waste or halogenated organic waste). The dry silica gel in nonhazardous solid waste.
b.
10 mL of unused 10% aqueous KOH solution
neutralize with aq 10 % HCl and flush down the drain with plenty of water.
c.
a clean but broken Erlenmeyer flask
(blue) broken glass trash can at end of bench
d.
The organic liquid residue left in the distilling flask after distillation dissolve in acetone and transfer to liquid organic hazardous waste
2.
Consider the reaction below that is sometimes performed in organic teaching labs to teach the principles of TLC, column chromatography, and melting points. The reaction produces a mixture of starting material (fluorene) and product (fluorenone) which can be easily separated by column chromatography.
Na2Cr2O7 CH3CO2H Fluorene, mp 114 oC, MW 166.2 O Fluorenone, mp 83 oC, MW 180.21
a.
If the reaction produced approximately 8 g of crude product to be purified by column chromatography, how much silica gel would be appropriate for the separation? 10-20 X +3 pts i. 8 g ii. 20 g iii. 120 g iv. 1200 g
b.
Unlike ferrocene and acetylferrocene, fluorene and fluroenone are both colorless, white solids. How would progress of the column chromatography be followed since no colored bands would be visible? +4 pts Collect fractions and TLC each fraction. Use some means to visualize the spots. Iodine or UV light work work. (Students could suggest others).
_____ 15 pts
1
Chemistry 2311, Fall 2008
Na 2Cr2O7 Fluorene, mp 114 oC, MW 166.2 CH 3CO2H O Fluorenone, mp 83 oC, MW 180.21
c.
If 8.0 g of fluorene was used in the reaction as the limiting reagent and after chromatographic separation, 4.7 g of fluorenone was isolated, what was the % yield of the reaction? Show all calculations. 6 pts 8.0 g fluorene/166.2 g/mol fluorene = 0.048 mol +2 0.048 mol x 180.21 g/mol fluorenone = 8.7 g theoretical yield of fluorenone +2 (can be combined with above) 4.7 g/8.7 x 100 = 54% yield +2
d.
Which compound would most likely elute from the column first? Circle one. 2 pts Fluorene Fluorenone
e.
Consider the 8 cm TLC plate below where fractions 4-11 were combined for spot A, fractions 1215 were combined for spot B, and fractions 16-25 were combined for spot C. Label the following on the TLC plate: pure fluorene, pure fluorenone. Calculate the Rf value for spot A using the given numbers (note the baseline starts at 0.6 cm not 0 cm) 8 pts
8.0 cm
7.5 cm
3.7-0.6 = 0.45 +4
5.9 cm 3.7 cm
+2 +2
Rf of Spot A =
7.5-0.6
A fluorene
C fluorenone
0.6 cm 0.0 cm
f.
A solid was isolated from spot B (fractions 12-15) and its melting point determined. Based on the TLC and given mpts of fluorene and fluorenone as 114 oC and 83 oC, respectively, which melting pt range below would most likely be observed (assume A is less than 50% of the sample B)? 3 pts a. 196-197 oC b. 90-95 oC c. 70-80 oC d. 82-83 oC
cant be higher than both or sum cant be higher than B if mostly B this is best guess since B can not be sharp if mixture
g.
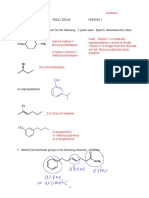
Which compound would have a singlet at 3.889 in the 1H NMR spectrum? Identify the Hs on the structure. 3 pts
Fluorene H H both benzylic so shifted downfield O Fluorenone no Hs not in 7-8 region
_____ 22 pts
2
Chemistry 2311, Fall 2008
3.
Simple Distillation - Briefly answer the following questions concerning a proper distillation. 12 pts a. Use the diagram of a distillation head below to show the proper positioning of the thermometer bulb for an accurate boiling point. +3
+3
b.
How is the size of the distilling flask chosen? not more t han 1/ 2 f ull of liquid t o be distilled What is the purpose of the boiling stones? +3
c.
prevent bumping - pr ov ide a surf ace f or escape of v apors prevent super heating ( all OK ) d. Why should a distillation be stopped bef ore it reaches dryness? f ormation of explosive peroxides or other materials may +3 be concentrated or discomposed residue left in glassware
4.
a.
Draw structures for each of the solvents below that you have used in lab. Circle the solvent that is the most polar. 8 pts ethyl acetate methylene chloride diethyl ether
CH 3CO2CH 2CH 3
CH 2Cl2
CH 3CH2OCH2CH3
5.
Extraction. During a separatory funnel extraction using diethyl ether and water, in which layer would you find the majority of each of the following compounds. Put O for organic layer, and A for aqueous layer. 5 pts
O A O A K O Cl O hexane O
NaHCO3
6.
Fischer Esterification Reaction After performing the banana oil synthesis in 2311 lab, a student was so enthused that she wanted to use the Fischer Esterification reaction to synthesize isobutyl propionate A, which has the flavor of rum. Give the starting materials and reagent(s) the student would need to perform this synthesis. 5 pts
+1 O CH 3CH2 C OH +2 +
HO CH 2CH(CH3)2 +2
H 2SO4
or any acid CH3CH 2 (catalyst)
O C O A
CH 3 CH 2CH CH 3
_____ 30 pts
3
Chemistry 2311, Fall 2008
7.
Standard Lab Techniques - In succinct words explain why 8 pts (+2 each) a. A heating mantle is not placed directly on the bench top during reflux.
So that the heat source can be quickly removed if necessary b. Pipetting directly from a reagent bottle is poor technique.
Contamination of the reagent can occur c. It is important to know the density of organic liquids before performing an extraction with water.
So that the top and bottom layer can be identified
d.
It is important to understand the mechanism of a reaction before performing it in lab.
So that by-products can be anticipated. Knowing if any gases are formed or physical properties of any by-products that would need to be separated or observed during purification such as distillation (TAs use your judgement on a good answer)
The End Please sign. I agree that discussing this quiz with any other student in the 2311 lab course will be detrimental to my grade. Therefore I promise to keep the contents and format of this quiz confidential until the TA has graded and returned the quiz. Signature _____________________________________
2 Bonus Points: Observe that the melting point of fluorene in problem 2 is HIGHER than the mpt of fluorenone, even though fluorenone is higher in polarity and molecular weight. What principle of melting point theory can account for this observation? Symmetry of crystal packing has a large influence on mpt. (Tightness of crystal lattice) Fluorene is more symmetrical than fluorenone.
_____ 8 + 2 Bonus points
4
You might also like
- TestFinal 350 v1 AnswersDocument8 pagesTestFinal 350 v1 AnswersJabe KoyNo ratings yet
- Stereochemistry Worksheet LabDocument3 pagesStereochemistry Worksheet LabDaniel McDermott0% (1)
- CMP Final Draft FuelDocument33 pagesCMP Final Draft Fuelapi-594648232No ratings yet
- Chapter 21 Further Aspects of EquilibriaDocument6 pagesChapter 21 Further Aspects of EquilibriaAndrea MelissaNo ratings yet
- CHEM1280 2012 13 Midterm Exam Solution PDFDocument5 pagesCHEM1280 2012 13 Midterm Exam Solution PDFLouisNo ratings yet
- 4b Evidence For Chemical Reactions LabDocument3 pages4b Evidence For Chemical Reactions Labapi-369690183100% (1)
- Chem1AA3 Lecture 1 PDFDocument110 pagesChem1AA3 Lecture 1 PDFbhavanjeetNo ratings yet
- Unit 8 EM MCQ Hydrocarbons 1991-2017Document24 pagesUnit 8 EM MCQ Hydrocarbons 1991-2017Imalka NanayakkaraNo ratings yet
- Chemistry Practice TestDocument2 pagesChemistry Practice Testyo mamaNo ratings yet
- Physics ReviewerDocument4 pagesPhysics ReviewermeriiNo ratings yet
- CH 2 Sec 1 Measurement-PowerpointDocument23 pagesCH 2 Sec 1 Measurement-Powerpointapi-294483847100% (2)
- MCQ Practice On (Chapter-3: Chemistry 1 Paper)Document4 pagesMCQ Practice On (Chapter-3: Chemistry 1 Paper)Mahin AzizNo ratings yet
- Exam 1 AnswersDocument9 pagesExam 1 AnswersArvic Lacson0% (1)
- Chemistry 2016 ExamsDocument20 pagesChemistry 2016 ExamsHoàng MinhNo ratings yet
- 11 Chemistry of Urine 1Document7 pages11 Chemistry of Urine 1Harold ApostolNo ratings yet
- Organic Chemistry TestDocument1 pageOrganic Chemistry Testron971No ratings yet
- 102 MSJC 13Document11 pages102 MSJC 13noelNo ratings yet
- Organic As Test P-2Document9 pagesOrganic As Test P-2zafarchem_iqbalNo ratings yet
- Flame TestsDocument4 pagesFlame TestsFelicia CharlesNo ratings yet
- Chem 132 2019 Tutorial QuestionsDocument3 pagesChem 132 2019 Tutorial QuestionsYusuf Zaynab100% (1)
- Inorganic Chemistry II (100 Items)Document11 pagesInorganic Chemistry II (100 Items)maria jeusa matiasNo ratings yet
- Mass Spectrometry (Multiple Choice) QPDocument12 pagesMass Spectrometry (Multiple Choice) QPYusuf AlamNo ratings yet
- Sample ProblemsDocument48 pagesSample Problemsapi-3856754No ratings yet
- Quiz 2 - Measurements and DensityDocument4 pagesQuiz 2 - Measurements and DensityCarolyn CampitaNo ratings yet
- 10 Lattice Energy - SDocument5 pages10 Lattice Energy - SisabelleNo ratings yet
- Unit-1 - AN OVERVIEW OF FOOD ChemistryDocument12 pagesUnit-1 - AN OVERVIEW OF FOOD ChemistryRadwan Ajo100% (1)
- PRACTICE MCQ HYDROCARBONS - 11ScADocument7 pagesPRACTICE MCQ HYDROCARBONS - 11ScAArda Rahmaini100% (1)
- Spectroscopy and ChromatographyDocument7 pagesSpectroscopy and ChromatographyPa GesNo ratings yet
- Organic Chemistry 2 Practice Exam 1Document15 pagesOrganic Chemistry 2 Practice Exam 1KaybidoNo ratings yet
- Worksheet 15 (CH 12, 13, 14)Document3 pagesWorksheet 15 (CH 12, 13, 14)Faheem ErshadNo ratings yet
- Chemistry 2 Answer KeyDocument8 pagesChemistry 2 Answer KeyMarielle BuesingNo ratings yet
- Atomic Structure: Examples of Multiple Choice QuestionsDocument4 pagesAtomic Structure: Examples of Multiple Choice Questionsngah lidwineNo ratings yet
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- Titrations in Analytical ChemistryDocument5 pagesTitrations in Analytical ChemistryBarronAliShapiNo ratings yet
- PLTL Ch. 16 AssignmentDocument6 pagesPLTL Ch. 16 AssignmentJules BrunoNo ratings yet
- Chemistry QuizDocument2 pagesChemistry Quizanon_572243106No ratings yet
- Carbenes and Nitrenes by IIT CampusDocument13 pagesCarbenes and Nitrenes by IIT CampusHemant soni100% (1)
- Chapter 3 - CALCULATIONS WITH CHEMICAL FORMULASDocument24 pagesChapter 3 - CALCULATIONS WITH CHEMICAL FORMULASSai RaghavaNo ratings yet
- 07 Introduction To Organic ChemistryDocument28 pages07 Introduction To Organic ChemistryM BNo ratings yet
- APCHEM Review Practice Test 1Document16 pagesAPCHEM Review Practice Test 1M. JosephNo ratings yet
- All Year Chemistry Up To 2018 PDFDocument37 pagesAll Year Chemistry Up To 2018 PDFAGAH LUCKYNo ratings yet
- 2013 As Chemistry Chapter 11 Review - GASESDocument11 pages2013 As Chemistry Chapter 11 Review - GASESbiancae697No ratings yet
- Stereo Chemistry QuestionsDocument19 pagesStereo Chemistry QuestionsfritzNo ratings yet
- Acid Base SolutionsDocument10 pagesAcid Base SolutionsCasey SangalliNo ratings yet
- ICSE Chemistry Board Paper19 PDFDocument9 pagesICSE Chemistry Board Paper19 PDFPrajakta DigheNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- CH 07Document96 pagesCH 07Jacquot AbendrothNo ratings yet
- MCQ On Periodic Classification of ElementsDocument2 pagesMCQ On Periodic Classification of ElementsNanda Rani SenNo ratings yet
- Hrushikesh Organic Group 5Document10 pagesHrushikesh Organic Group 5Sarita YadavNo ratings yet
- 2013 Organic Chemistry Exam First YearDocument7 pages2013 Organic Chemistry Exam First YearAnonymous oqlnO8eNo ratings yet
- Module8 PDFDocument40 pagesModule8 PDFFaizan AhmadNo ratings yet
- Alkenes and Alkynes II:: Addition ReactionsDocument102 pagesAlkenes and Alkynes II:: Addition Reactionsfingil20032003No ratings yet
- Chemical Reactions: John A. Schreifels Chemistry 211-Notes 1Document22 pagesChemical Reactions: John A. Schreifels Chemistry 211-Notes 1Hayan LeeNo ratings yet
- Applied Chemistry MCQsDocument10 pagesApplied Chemistry MCQsiangarvins100% (1)
- Class Test 1: Section A (Multiple-Choice Questions)Document10 pagesClass Test 1: Section A (Multiple-Choice Questions)Kgaugelo TraciaNo ratings yet
- Surface Chemistry MCQs - Questions - Paper 1Document7 pagesSurface Chemistry MCQs - Questions - Paper 1krishna prasad ghantaNo ratings yet
- 1.intro To Organic Chem-PracticeDocument2 pages1.intro To Organic Chem-PracticeZul Abror Bin Ya'akopNo ratings yet
- 19.2 Acid-Base Titration CurvesDocument9 pages19.2 Acid-Base Titration CurvesYuyun Sri IriantiNo ratings yet
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisFrom EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisRating: 4 out of 5 stars4/5 (2)
- Lab 5 Lab Plant Pigments 3-23-09Document4 pagesLab 5 Lab Plant Pigments 3-23-09Bayu PutraNo ratings yet
- Phloxine BDocument7 pagesPhloxine BDeepa DevanathanNo ratings yet
- 80010768Document11 pages80010768ArmanNo ratings yet
- Ethics of Student Final PDFDocument15 pagesEthics of Student Final PDFAmtul KafiNo ratings yet
- SkincareDocument84 pagesSkincareNikoletta AslanidiNo ratings yet
- Int Er Ene Rgy: Ie210 210/M/55/MHDocument2 pagesInt Er Ene Rgy: Ie210 210/M/55/MHRusnacNo ratings yet
- Military Engineer Services Notice Inviting Tender (Nit)Document14 pagesMilitary Engineer Services Notice Inviting Tender (Nit)jatanNo ratings yet
- Class 9 GrammarDocument32 pagesClass 9 GrammarPerajothi PalanirajaNo ratings yet
- Ilovepdf MergedDocument144 pagesIlovepdf MergedChandra PrakashNo ratings yet
- Component Identification of GearDocument6 pagesComponent Identification of GearWandiNo ratings yet
- Tos - TrendsDocument2 pagesTos - TrendsBernadette Falceso100% (1)
- Consilium Navigation Sal Broschyr PDFDocument8 pagesConsilium Navigation Sal Broschyr PDFSeamen 777No ratings yet
- MSDS Enercept - Ec1304aDocument11 pagesMSDS Enercept - Ec1304aKader Rahmani100% (1)
- Notification No. 477 (E) Dated 25th July, 1991 of The Department of Industrial Policy and Promotion, Ministry of IndustryDocument39 pagesNotification No. 477 (E) Dated 25th July, 1991 of The Department of Industrial Policy and Promotion, Ministry of IndustryHarish100% (3)
- Top Survival Tips - Kevin Reeve - OnPoint Tactical PDFDocument8 pagesTop Survival Tips - Kevin Reeve - OnPoint Tactical PDFBillLudley5100% (1)
- Keep 425Document12 pagesKeep 425Johan SukweenadhiNo ratings yet
- Threat Modeling Tool 2016 User GuideDocument59 pagesThreat Modeling Tool 2016 User GuideSenthilkumarmoorthyNo ratings yet
- Annotated BibliographyDocument2 pagesAnnotated Bibliographyapi-254427588No ratings yet
- Clarinet GroveDocument38 pagesClarinet GroveLaMusica IlMio:nomeNo ratings yet
- Numerical ModellingDocument119 pagesNumerical Modellingjanusz_1025No ratings yet
- Android Quiz App 22617 MADDocument19 pagesAndroid Quiz App 22617 MADSandip kotkarNo ratings yet
- Digital Storage Oscilloscopes: TDS210 TDS220 TDS224Document4 pagesDigital Storage Oscilloscopes: TDS210 TDS220 TDS224Jime SiepeNo ratings yet
- Airburst - Hendrick.r17 LRDocument2 pagesAirburst - Hendrick.r17 LRManuel Javier Rodríguez VNo ratings yet
- Literature Review On Hospital Information SystemsDocument8 pagesLiterature Review On Hospital Information Systemsc5mr3mxfNo ratings yet
- Newborn Studio Guide by Jessica G. PhotographyDocument13 pagesNewborn Studio Guide by Jessica G. PhotographyClau ppNo ratings yet
- Steven Johnsons SyndromeDocument22 pagesSteven Johnsons SyndromeRoselene Mae MarasiganNo ratings yet
- Case Shaping Future Business Leaders 9 June 2016Document7 pagesCase Shaping Future Business Leaders 9 June 2016Alka AggarwalNo ratings yet
- 2004 Waziristan Complex Chem Data Interptretaion SHAKIRULLAH & MOHAMMAD IHSAN AFRIDIDocument16 pages2004 Waziristan Complex Chem Data Interptretaion SHAKIRULLAH & MOHAMMAD IHSAN AFRIDInoor abdullahNo ratings yet
- Performance Appraisal at Jindal Brothers Pvt. LTD: A Dissertation Report ONDocument50 pagesPerformance Appraisal at Jindal Brothers Pvt. LTD: A Dissertation Report ONAvinash ThakurNo ratings yet
- Rehab-Plans-and-Exercises Hip-Arthroscopy Protocol-For-Physiotherapy-Following-SurgeryDocument2 pagesRehab-Plans-and-Exercises Hip-Arthroscopy Protocol-For-Physiotherapy-Following-SurgeryMellow Moon RecordsNo ratings yet
- Jerkins The FounderDocument15 pagesJerkins The FounderRifqi Khairul AnamNo ratings yet