Professional Documents
Culture Documents
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Uploaded by
abdulrehman999Copyright:
Available Formats
You might also like
- Chemistry Matters Ch16 Textbk ANSDocument2 pagesChemistry Matters Ch16 Textbk ANSZeneon63% (8)
- Causes of Separation of East PakistanDocument27 pagesCauses of Separation of East Pakistanabdulrehman99969% (16)
- Benedict's Test For Non-Reducing SugarsDocument2 pagesBenedict's Test For Non-Reducing SugarsSamer Ehab75% (4)
- Benedict's Test For Reducing SugarDocument2 pagesBenedict's Test For Reducing SugarMohammed Parfals100% (2)
- Atomic AnswersDocument10 pagesAtomic AnswersKelumNo ratings yet
- ICSE Paper 2008Document8 pagesICSE Paper 2008CGPSC - P&P TutorialNo ratings yet
- 2021 F.3 Final ExamDocument6 pages2021 F.3 Final ExamUncomfortsNo ratings yet
- Chemistry Perfect Score 2011 Module AnswerDocument43 pagesChemistry Perfect Score 2011 Module Answersarahrozaimi100% (1)
- ICSE Paper 2008Document12 pagesICSE Paper 2008Geetansh KhuranaNo ratings yet
- Perfect Score Chemistry SBP 2012 - ANSWERDocument61 pagesPerfect Score Chemistry SBP 2012 - ANSWERAhmad RawiNo ratings yet
- Mark Scheme Summative Assessment - I Grade-7 ChemistryDocument3 pagesMark Scheme Summative Assessment - I Grade-7 ChemistryVivek Sadasivan NairNo ratings yet
- ICSE X SP 03 (Questions)Document10 pagesICSE X SP 03 (Questions)aadithlamjonlNo ratings yet
- UntitledDocument2 pagesUntitledAye Pyae SoneNo ratings yet
- Marking Scheme Paper ChemistryDocument20 pagesMarking Scheme Paper ChemistryArvin DiNozzoNo ratings yet
- 2008-Teacher 20080324 1509 2Document20 pages2008-Teacher 20080324 1509 2Mateo PremarionNo ratings yet
- Chemistry Paper II FinalDocument3 pagesChemistry Paper II FinalShaziaNo ratings yet
- Nonmetals and Metalloids: Examples of Multiple Choice QuestionsDocument20 pagesNonmetals and Metalloids: Examples of Multiple Choice Questionsngah lidwineNo ratings yet
- Chemistry 1 2013Document3 pagesChemistry 1 2013Tayyab ZafarNo ratings yet
- Atomic - Structure Review AnswersDocument7 pagesAtomic - Structure Review AnswersZain AhmadNo ratings yet
- Metals and Non Metals (Grand Test)Document10 pagesMetals and Non Metals (Grand Test)amit mongiaNo ratings yet
- QP 2452Document5 pagesQP 2452yashojayoneplusNo ratings yet
- D and F Block DPPDocument4 pagesD and F Block DPPKalyan ReddtNo ratings yet
- SS2 ChemistryDocument5 pagesSS2 ChemistrySUNDAY JAMESNo ratings yet
- Skema BK2 KimiaDocument12 pagesSkema BK2 KimiaazmiNo ratings yet
- 10th Chemistry Sample Paper 2Document7 pages10th Chemistry Sample Paper 2GURANSH DEEPNo ratings yet
- Work Sheet S Block ElementsDocument6 pagesWork Sheet S Block ElementsxxxxNo ratings yet
- O XO2 Ud DNUBLYl GZ WO7 QoDocument12 pagesO XO2 Ud DNUBLYl GZ WO7 QoPratyush MishraNo ratings yet
- Trial Paper 2 MS PerlisDocument8 pagesTrial Paper 2 MS PerlisZaiton RoslanNo ratings yet
- PDF YesterdayDocument352 pagesPDF Yesterdaysudhasingh162900No ratings yet
- (WWW - Entrance-Exam - Net) - ICSE Class 10 Chemistry Sample Paper 1Document12 pages(WWW - Entrance-Exam - Net) - ICSE Class 10 Chemistry Sample Paper 1Vaibhav66No ratings yet
- 10th Chapter 3 DPPs - Metals and Non-MetalsDocument12 pages10th Chapter 3 DPPs - Metals and Non-MetalsYash KapoorNo ratings yet
- Metals and Non-Metals: Multiple Choice QuestionsDocument10 pagesMetals and Non-Metals: Multiple Choice QuestionsShreyansh DuggarNo ratings yet
- 10th MCQ-QP AnswersDocument5 pages10th MCQ-QP AnswersNARENDRAN S0% (1)
- Electrochemistry Practice Test: (A) Loses ElectronsDocument5 pagesElectrochemistry Practice Test: (A) Loses ElectronsElla Canonigo CanteroNo ratings yet
- Part VII Redox Reactions, Chemical Cells and Electrolysis TestDocument11 pagesPart VII Redox Reactions, Chemical Cells and Electrolysis Testpallavi mirpuri cortésNo ratings yet
- Chapter 3 - ElectrochemistryDocument9 pagesChapter 3 - ElectrochemistryShubh MishraNo ratings yet
- Work Sheet For G10Document2 pagesWork Sheet For G10Firaol GeremuNo ratings yet
- Metals AnswersDocument11 pagesMetals AnswersKelumNo ratings yet
- SS 2 First Term Chemistry ExaminationDocument8 pagesSS 2 First Term Chemistry ExaminationUzoma ObasiNo ratings yet
- Paper 1 Chem ICSEDocument4 pagesPaper 1 Chem ICSEAkash KaleNo ratings yet
- ICSE Class 8 Chemistry Sample Paper 2Document6 pagesICSE Class 8 Chemistry Sample Paper 2Naman GuptaNo ratings yet
- Icse Test 2Document4 pagesIcse Test 2RAM KUMARNo ratings yet
- Topper 8 110 2 2 Chemistry 2008 Questions Up201506182058 1434641282 7298Document7 pagesTopper 8 110 2 2 Chemistry 2008 Questions Up201506182058 1434641282 7298Manohar GarimellaNo ratings yet
- Periodic Table Multiple Choice Questions: Answer SheetDocument14 pagesPeriodic Table Multiple Choice Questions: Answer SheetlionelkenethNo ratings yet
- Chemistry FigureDocument5 pagesChemistry FigureSalim AllyNo ratings yet
- Topper 2 110 7 2 Chemistry Question Up201711171822 1510923166 8286Document7 pagesTopper 2 110 7 2 Chemistry Question Up201711171822 1510923166 8286UMANo ratings yet
- Chemistry IMU CET PDFDocument64 pagesChemistry IMU CET PDFAniket KNo ratings yet
- Alphonsa School, Kalamjote - Preboard - ChemistryDocument4 pagesAlphonsa School, Kalamjote - Preboard - Chemistryakshayashivakumar96No ratings yet
- ElecrochemistryDocument7 pagesElecrochemistryffxfuddiNo ratings yet
- Chemistry - 9 Time: 2 Hours M.M. 80: Section I (40 Marks) Attempt All Questions From This SectionDocument4 pagesChemistry - 9 Time: 2 Hours M.M. 80: Section I (40 Marks) Attempt All Questions From This SectionGreatAkbar1No ratings yet
- Https Doc 0c 0c Apps Viewer - GoogleusercontentDocument9 pagesHttps Doc 0c 0c Apps Viewer - GoogleusercontentAhmad RezaNo ratings yet
- Chapter 3 Electrochemistry Topic ElectrochemistryDocument16 pagesChapter 3 Electrochemistry Topic Electrochemistryvivek daveNo ratings yet
- 2003 Csec Chem Paper 01Document10 pages2003 Csec Chem Paper 01Jesshaun Morris100% (6)
- Karnataka Icse Schools Association: SECTION A (40 Marks)Document6 pagesKarnataka Icse Schools Association: SECTION A (40 Marks)Arebal100% (1)
- Eje Islamic f4 22 Chem 1-1Document7 pagesEje Islamic f4 22 Chem 1-1Nassrah JumaNo ratings yet
- Delhi Public School Newtown SESSION: 2021-22 Final Term Examination Class: Ix Total Marks: 80 Subject: Chemistry Time: 2 HoursDocument7 pagesDelhi Public School Newtown SESSION: 2021-22 Final Term Examination Class: Ix Total Marks: 80 Subject: Chemistry Time: 2 HoursSAMPURNA GHOSHNo ratings yet
- ElectrolysisDocument6 pagesElectrolysisskylar chanNo ratings yet
- Test - XII - 21.11.2023 - D & F Block Elements & OrganicDocument6 pagesTest - XII - 21.11.2023 - D & F Block Elements & Organicsaanvi2629jindalNo ratings yet
- IMUCET PCM CombinedDocument193 pagesIMUCET PCM Combinedshuklaity01No ratings yet
- Definition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-HomogeneousDocument8 pagesDefinition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-Homogeneousabdulrehman999No ratings yet
- Mit18 06scf11 Ses2.5sumDocument4 pagesMit18 06scf11 Ses2.5sumabdulrehman999No ratings yet
- Pureit Excella User Manual PDFDocument31 pagesPureit Excella User Manual PDFabdulrehman999No ratings yet
- Social ScienceDocument4 pagesSocial Scienceabdulrehman999No ratings yet
- Practical 12 Object: Tasks: Working With Arrays in C++Document6 pagesPractical 12 Object: Tasks: Working With Arrays in C++abdulrehman999No ratings yet
- Step-by-Step Calculator: Cosx Sin XDocument4 pagesStep-by-Step Calculator: Cosx Sin Xabdulrehman999No ratings yet
- Practical-8 To 10Document18 pagesPractical-8 To 10abdulrehman999No ratings yet
- Practical # 07 (16EL84)Document9 pagesPractical # 07 (16EL84)abdulrehman999No ratings yet
- English Grammar - LearnEnglish - British Council - Sentence StructureDocument12 pagesEnglish Grammar - LearnEnglish - British Council - Sentence Structureabdulrehman999No ratings yet
- Cost Accounting Mcqs - PaperpkinfoDocument8 pagesCost Accounting Mcqs - Paperpkinfoabdulrehman999100% (2)
- Chemistry SPM Potential Questions-Form5chap1 2Document15 pagesChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNo ratings yet
- Benzoic Acid From TolueneDocument66 pagesBenzoic Acid From TolueneIgnacio Real BuffelliNo ratings yet
- Canadian Business English Canadian 7th Edition Guffey Solutions ManualDocument35 pagesCanadian Business English Canadian 7th Edition Guffey Solutions Manualpeanutsofteniscd1n100% (31)
- Crude OilDocument8 pagesCrude OilAathifa ThowfeekNo ratings yet
- Chem (Final)Document17 pagesChem (Final)Jaynie Lee VillaranNo ratings yet
- Wet EtchingDocument15 pagesWet Etchingnskprasad89No ratings yet
- Organic Distinguishing Tests NotesDocument1 pageOrganic Distinguishing Tests Notesihshan007No ratings yet
- Baker Safe-T-DataDocument1 pageBaker Safe-T-DataJonathan Saviñon de los SantosNo ratings yet
- Preparation and Reactions of Boric Acid, H3BO3Document8 pagesPreparation and Reactions of Boric Acid, H3BO3Sin YeeNo ratings yet
- Product Catalogue 2011 12Document184 pagesProduct Catalogue 2011 12Jagesh RanjanNo ratings yet
- The Boron FamilyDocument27 pagesThe Boron Familygautambadgujar30No ratings yet
- Core (STEM) - EarthScience-SLMG11Q1W1-Identify Common Rock-Forming Minerals Using Their Physcial and Chemical PropertiesDocument18 pagesCore (STEM) - EarthScience-SLMG11Q1W1-Identify Common Rock-Forming Minerals Using Their Physcial and Chemical PropertiesRM L. JamandronNo ratings yet
- Class 12 Chemistry Set 1Document15 pagesClass 12 Chemistry Set 1latestdaaNo ratings yet
- Latihan NMR Dan FT-IRDocument37 pagesLatihan NMR Dan FT-IRWidi KurniaNo ratings yet
- Syllabus IOC I Pharm.D (C.L Baid)Document4 pagesSyllabus IOC I Pharm.D (C.L Baid)giridharan rajendranNo ratings yet
- Preservative Selection GuideDocument14 pagesPreservative Selection Guidedecker.19369No ratings yet
- TitrationDocument23 pagesTitrationAKSHAY MISHRA100% (1)
- Chapter 5 ConclusionDocument4 pagesChapter 5 ConclusionRaffandi RolandoNo ratings yet
- CHE 321 - CH5 - Fatty AlcoholDocument27 pagesCHE 321 - CH5 - Fatty AlcoholnorazifahNo ratings yet
- ACGDocument10 pagesACGrishichauhan25No ratings yet
- F-720-032 Rev. B O-Ring KitsDocument2 pagesF-720-032 Rev. B O-Ring KitsRD Rental CampoNo ratings yet
- Complejos de Cobalto ArtDocument3 pagesComplejos de Cobalto ArtNatalia MayaNo ratings yet
- Reaction of AlkynesDocument25 pagesReaction of AlkynesGabrielaAmandaNo ratings yet
- 50% Caustic Soda Membrane West Coast: Sales SpecificationDocument1 page50% Caustic Soda Membrane West Coast: Sales SpecificationMeziane BouktitNo ratings yet
- PTA Technology - FundamentalsDocument50 pagesPTA Technology - FundamentalsSarat Na NiNo ratings yet
- Class 12 Book 6 Organic Chemistry BiomoleculesDocument17 pagesClass 12 Book 6 Organic Chemistry Biomoleculesfakeu foreverNo ratings yet
- PPDDocument79 pagesPPDEddie ChangNo ratings yet
- Structured Questions: HKDSE Chemistry A Modern View Part X Chemical EquilibriumDocument26 pagesStructured Questions: HKDSE Chemistry A Modern View Part X Chemical EquilibriumNg Swee Loong StevenNo ratings yet
- CrystalDocument2 pagesCrystalAduchelab AdamsonuniversityNo ratings yet
- Optibor: Product Data SheetDocument5 pagesOptibor: Product Data Sheetanon_993394650No ratings yet
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Uploaded by
abdulrehman999Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Gcse Chemistry Answers and Mark Schemes: The Periodic Table
Uploaded by
abdulrehman999Copyright:
Available Formats
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 1
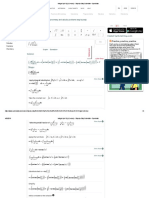
(a) (i) (ii) (b) (i) (ii) noble/inert/rare gases two out of helium, neon, argon, krypton, xenon, radon. C atoms do not combine/monatomic atoms/inert gas so atoms dont join/ A, B and C show molecules and group 0/8 elements do not form molecules 1 2 1
1 1 1 1 1 1
(d)
(i) (ii) (iii)
all unreactive/stays the same density increases all non metals/stays the same measure the density of each
(e)
(iv)
ctiv
e.c ma
om
(c)
splint goes out/does not burn
TOTAL 10
QUESTIONSHEET 2
(a) (i) (ii) A
1 1 1 1 1 1
(iii) (b) (i) (ii) (c)
one electron in outside shell
charged atom/group of atoms K+
Any two from: very reactive react with air react with oxygen in the air react with water in the air (i) (ii) (iii) (iv) oxygen (in the air) potassium oxide
ww w.c
he
A has 11 electrons and atomic number of sodium is 11 A has one electron in outside shell but ions have full outer shell
2 1 1 1 2
(d)
potassium + oxygen potassium oxide 4K + O2 2K2O
TOTAL 13
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 3
(a) (i) (ii) (b) (i) (ii) (iii) (c) lithium + chlorine lithium chloride 2Li + Cl2 2LiCl group 1 1 7 x 1 1 1 1
om
+ C1 Li
+ x
Li
C1
(d)
(i) (ii)
more reactive common/table/cooking salt
ctiv
diagram to show: chlorine seven electrons lithium one electron being transferred to chlorine lithium ion one positive charge, chloride ion one negative charge
e.c
1 1 1 1 1 TOTAL 10
ww w.c
he
ma
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 4
(a) (i) (ii) (iii) (iv) (b) (i) (ii) (iii) (iv) (v) (vi) (c) (i) ions sodium ion chloride ion attraction of opposite charges/ electrostatic attraction 1 7 3 3 1 7 1 1 1 1
ctiv
e.c
om
6 1 1 1 1
x Na
(ii)
one sodium ion electrically balances one chloride ion
he
C1
Na+
ma
x
sodium needs to lose one electron to empty outside shell/ to give full/complete outer shell/octet/stable electron arrangement sodium transfers electron to chlorine atom which completes its octet
C1
ww w.c
TOTAL 16
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 5
(a) (i) (ii) (b) (i) (ii) (iii) (c) (i) (ii) (iii) (iv) to kill bacteria/germs in water antiseptic/put on cuts to kill bacteria/germs halogens non-metal non-metal 17 protons in nucleus/17 electrons in uncombined atom 3 7 sodium iodide NaI 1 1 1 1 1
om
1 1 1 1 1 TOTAL 10
QUESTIONSHEET 6
(a) (i) (ii) (b) (i) (ii) (c) (i) (ii) goes dark photosynthesis/colour fading ion/anion/negative ion no go dark
ma
ctiv
e.c
1 1 1 1 1 3
Ag+(aq) + I (aq) AgI(s)
(2 marks for correct equation; only 1 if ions not shown. 1 mark for state symbols)
ww w.c
-
he
TOTAL 8
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 7
(a) (i) (ii) (iii) isotopes different number of neutrons number of protons number of electrons in uncombined atom 35 (neutrons + protons) 17 (protons) 18 neutrons mixture if different isotopes/chlorine-35 and chlorine-37 (i) (ii) no difference 1 1 1 1 1 1
(iv)
(b) (c)
om
1 1
Any two from: chemical properties stay the same both elements have 7 electrons/same number of electrons in their outside shell chemical properties depend on number of electrons in outside shell no
e.c
2 1 1 TOTAL 12
(iii) (iv)
different masses/physical properties depend on the mass
(a)
(i) (ii)
he
QUESTIONSHEET 8
ma
ctiv
1 1 1 1 1 1
second electron shell is full next electron has to go into third shell/one electron in outside shell sodium, magnesium and aluminium/Na, Mg and Al (all three needed for mark)
(b)
(i) (ii)
go from metallic (left hand side) to non-metallic (right hand side) 6 (electrons)
(c)
(i) (ii)
sodium oxide: Na2O magnesium oxide: MgO aluminium oxide: Al2O3
ww w.c
3 TOTAL 9
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 9
(a) (b) (i) (ii) group 0/8 / noble gases/inert gases drop/go down/fall Any two from: lower down in the group group 0/8 elements get denser as the atomic number increases krypton denser/heavier for the same volume than the gases in the other balloons/air
x
1 1
(c)
(i)
om
(ii)
outer shell complete helium does not combine/react with anything
(ii)
unreactive/inert/full outer electron shells wont react with hot filament of light bulb
ctiv
(d)
(i)
very light/less dense than air unreactive/does not react with air/non-flammable
e.c
1 1 1 1 1 1 1
ma
TOTAL 11
QUESTIONSHEET 10
(a) (i) (ii) (iii) (b) group 1 alkali metals white
he
1 1 1
Any three from: use platinum/nichrome wire clean wire/by dipping in hydrochloric acid and putting into a hot flame dip wire into salt/solution of salt/hydrochloric acid and then salt put into blue flame/hottest part of flame/side of a blue flame look at colour of flame. A = sodium chloride B = lithium chloride C = potassium chloride
ww w.c
3 1 1 1
(c)
TOTAL 9
GCSE CHEMISTRY QUESTIONSHEET 11
(a) (i) (ii) (b) (i) (ii) (iii)
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
chlorine, bromine, iodine atomic number 7 molecules Any two from: outer shell incomplete each chlorine atom needs one more electron to complete its octet each atom shares one electron
xx x x x xx x x
1 1 1 1
om
(c)
(i)
(ii) (iii) (d)
gas -220 C (accept 200 C to 250 C) boils/becomes a gas/vapour
o o o
ctiv
diagram to show: two electron shells two electrons in first shell, seven in outside shell
e.c
1 1 1 1 1
he
ma
TOTAL 11
QUESTIONSHEET 12
(a) (i) (ii) colourless
ww w.c
Any two from: a solid in a liquid very small particles of solid. silver chloride
2 1 1 1 1 2 2 TOTAL 11
(iii) (b) (i) (ii) (iii) (iv) (c)
silver nitrate + sodium chloride silver chloride + sodium nitrate (aq) (s) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) B and C
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 13
(a) (i) (ii) (iii) (b) (i) (ii) transition elements/ transition metals metals Fe = iron, Cu = copper, Zn = zinc A A high melting point/B would melt when the water boiled hard can be pulled out to make a wire shiny surface that can be polished 1 1 3 1 1 1 1 1 1 TOTAL 11
(iii)
QUESTIONSHEET 14
(a) (i)
one out of scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, zinc, or between atomic numbers 39-48, 57, 72-80 including silver, gold, platinum and mercury limewater
ctiv
e.c
om
1 1 1 1 1 1 1 1 1 1
(ii) (iii) (iv) (b) (i) (ii)
lime water went cloudy/milky/white precipitate
copper(II) oxide
gas made copper carbonate changed colour
(iii) (iv) (c) (i) (ii)
copper carbonate copper oxide + carbon dioxide CuCO3 CuO + CO2 B
Any two from: there was a reaction in B/no reaction in A zinc carbonate/transition metal carbonate breaks down on heating sodium carbonate/group 1 metal carbonates do not break down on heating zinc oxide is yellow when hot and white when cold Any two from: A was sodium carbonate sodium carbonate does not break down on heating group 1 metal carbonates do not break down on heating
ww w.c
he
carbon dioxide
ma
(iii)
TOTAL 14
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 15
(a) (i) (ii) (b) (i) transition elements blue/green add a small amount of sodium hydroxide solution colour of precipitate indicates which metal ion is present A = copper B = green C = iron(III) (all three for 2 marks one or two for one mark) Fe2+ OHFe2+ + 2OH- Fe(OH)2 1 1 1 1
(ii)
om
(c)
(i) (ii) (iii)
1 1 1
ctiv
e.c ma
TOTAL 9
QUESTIONSHEET 16
(a) (i) (ii) (iii) (b) (i) (ii) (iii) (c) (i) 2 8
1 1 1 1 1 1 1 1 1 1
the number of electron shells 2 4
the number of electrons in the outside shell 6 protons in atom/ in the nucleus 6 electrons in uncombined atom
(ii) (iii)
mass number/ relative atomic mass
number of neutrons plus number of protons
ww w.c
he
TOTAL 10
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 17
(a) (i) (ii) (iii) (b) (i) (ii) (iii) (iv) (c) (d) (i) (ii) (iii) One out of: Li, Be, Na, Mg, Al, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge. One out of: H, N, O, F, Cl, He, Ne, Ar, Kr. One out of: Ti, V, Cr, Mn, Fe, Co, Ni, Cu. (allow Sc and Zn) 7 3 3 7 1 1 1 1 1
om
1 1 1 1 1 1
number of protons/number of electrons in neutral (uncombined) atom inert/noble/rare gases unreactive/do not react
outer electron shell contains 8 electrons/ complete octet
ma
ctiv
e.c
TOTAL 11
QUESTIONSHEET 18
(a) (i) (ii) (iii) (b) (c) (i) (ii) (d) (i) (ii) (e) lithium rubidium potassium blue
he
1 1 1 1 1 1 1 1
burnt with a pop/small explosion hydrogen
more reactive
hydrogen and sodium hydroxide Any two from: react with water to give alkalis all metals hydroxides are alkalis hydroxides all dissolve in water to give alkaline solutions
ww w.c
2 TOTAL 10
GCSE CHEMISTRY
THE PERIODIC TABLE ANSWERS AND MARK SCHEMES
QUESTIONSHEET 19
(a) (i) Any two from: high melting point good conductors of heat strong/high tensile strength expensive/copper compounds are poisonous good conductor of electricity ductile/high tensile strength cars: strong/high tensile strength/(relatively) cheap planes: high density speeds up a reaction with transition elements positive/cation Cu2+ Fe3+
2 1 1 1 1 1 1 1 1 2
(ii) (iii)
(iv)
(b)
(i) (ii)
(c)
(i) (ii)
ma
ctiv
e.c he
om
TOTAL 12
QUESTIONSHEET 20
(i) I II III IV V VI VII C or E F or A A D D G E
ww w.c
1 1 1 1 1 1 1
TOTAL 7
You might also like
- Chemistry Matters Ch16 Textbk ANSDocument2 pagesChemistry Matters Ch16 Textbk ANSZeneon63% (8)
- Causes of Separation of East PakistanDocument27 pagesCauses of Separation of East Pakistanabdulrehman99969% (16)
- Benedict's Test For Non-Reducing SugarsDocument2 pagesBenedict's Test For Non-Reducing SugarsSamer Ehab75% (4)
- Benedict's Test For Reducing SugarDocument2 pagesBenedict's Test For Reducing SugarMohammed Parfals100% (2)
- Atomic AnswersDocument10 pagesAtomic AnswersKelumNo ratings yet
- ICSE Paper 2008Document8 pagesICSE Paper 2008CGPSC - P&P TutorialNo ratings yet
- 2021 F.3 Final ExamDocument6 pages2021 F.3 Final ExamUncomfortsNo ratings yet
- Chemistry Perfect Score 2011 Module AnswerDocument43 pagesChemistry Perfect Score 2011 Module Answersarahrozaimi100% (1)
- ICSE Paper 2008Document12 pagesICSE Paper 2008Geetansh KhuranaNo ratings yet
- Perfect Score Chemistry SBP 2012 - ANSWERDocument61 pagesPerfect Score Chemistry SBP 2012 - ANSWERAhmad RawiNo ratings yet
- Mark Scheme Summative Assessment - I Grade-7 ChemistryDocument3 pagesMark Scheme Summative Assessment - I Grade-7 ChemistryVivek Sadasivan NairNo ratings yet
- ICSE X SP 03 (Questions)Document10 pagesICSE X SP 03 (Questions)aadithlamjonlNo ratings yet
- UntitledDocument2 pagesUntitledAye Pyae SoneNo ratings yet
- Marking Scheme Paper ChemistryDocument20 pagesMarking Scheme Paper ChemistryArvin DiNozzoNo ratings yet
- 2008-Teacher 20080324 1509 2Document20 pages2008-Teacher 20080324 1509 2Mateo PremarionNo ratings yet
- Chemistry Paper II FinalDocument3 pagesChemistry Paper II FinalShaziaNo ratings yet
- Nonmetals and Metalloids: Examples of Multiple Choice QuestionsDocument20 pagesNonmetals and Metalloids: Examples of Multiple Choice Questionsngah lidwineNo ratings yet
- Chemistry 1 2013Document3 pagesChemistry 1 2013Tayyab ZafarNo ratings yet
- Atomic - Structure Review AnswersDocument7 pagesAtomic - Structure Review AnswersZain AhmadNo ratings yet
- Metals and Non Metals (Grand Test)Document10 pagesMetals and Non Metals (Grand Test)amit mongiaNo ratings yet
- QP 2452Document5 pagesQP 2452yashojayoneplusNo ratings yet
- D and F Block DPPDocument4 pagesD and F Block DPPKalyan ReddtNo ratings yet
- SS2 ChemistryDocument5 pagesSS2 ChemistrySUNDAY JAMESNo ratings yet
- Skema BK2 KimiaDocument12 pagesSkema BK2 KimiaazmiNo ratings yet
- 10th Chemistry Sample Paper 2Document7 pages10th Chemistry Sample Paper 2GURANSH DEEPNo ratings yet
- Work Sheet S Block ElementsDocument6 pagesWork Sheet S Block ElementsxxxxNo ratings yet
- O XO2 Ud DNUBLYl GZ WO7 QoDocument12 pagesO XO2 Ud DNUBLYl GZ WO7 QoPratyush MishraNo ratings yet
- Trial Paper 2 MS PerlisDocument8 pagesTrial Paper 2 MS PerlisZaiton RoslanNo ratings yet
- PDF YesterdayDocument352 pagesPDF Yesterdaysudhasingh162900No ratings yet
- (WWW - Entrance-Exam - Net) - ICSE Class 10 Chemistry Sample Paper 1Document12 pages(WWW - Entrance-Exam - Net) - ICSE Class 10 Chemistry Sample Paper 1Vaibhav66No ratings yet
- 10th Chapter 3 DPPs - Metals and Non-MetalsDocument12 pages10th Chapter 3 DPPs - Metals and Non-MetalsYash KapoorNo ratings yet
- Metals and Non-Metals: Multiple Choice QuestionsDocument10 pagesMetals and Non-Metals: Multiple Choice QuestionsShreyansh DuggarNo ratings yet
- 10th MCQ-QP AnswersDocument5 pages10th MCQ-QP AnswersNARENDRAN S0% (1)
- Electrochemistry Practice Test: (A) Loses ElectronsDocument5 pagesElectrochemistry Practice Test: (A) Loses ElectronsElla Canonigo CanteroNo ratings yet
- Part VII Redox Reactions, Chemical Cells and Electrolysis TestDocument11 pagesPart VII Redox Reactions, Chemical Cells and Electrolysis Testpallavi mirpuri cortésNo ratings yet
- Chapter 3 - ElectrochemistryDocument9 pagesChapter 3 - ElectrochemistryShubh MishraNo ratings yet
- Work Sheet For G10Document2 pagesWork Sheet For G10Firaol GeremuNo ratings yet
- Metals AnswersDocument11 pagesMetals AnswersKelumNo ratings yet
- SS 2 First Term Chemistry ExaminationDocument8 pagesSS 2 First Term Chemistry ExaminationUzoma ObasiNo ratings yet
- Paper 1 Chem ICSEDocument4 pagesPaper 1 Chem ICSEAkash KaleNo ratings yet
- ICSE Class 8 Chemistry Sample Paper 2Document6 pagesICSE Class 8 Chemistry Sample Paper 2Naman GuptaNo ratings yet
- Icse Test 2Document4 pagesIcse Test 2RAM KUMARNo ratings yet
- Topper 8 110 2 2 Chemistry 2008 Questions Up201506182058 1434641282 7298Document7 pagesTopper 8 110 2 2 Chemistry 2008 Questions Up201506182058 1434641282 7298Manohar GarimellaNo ratings yet
- Periodic Table Multiple Choice Questions: Answer SheetDocument14 pagesPeriodic Table Multiple Choice Questions: Answer SheetlionelkenethNo ratings yet
- Chemistry FigureDocument5 pagesChemistry FigureSalim AllyNo ratings yet
- Topper 2 110 7 2 Chemistry Question Up201711171822 1510923166 8286Document7 pagesTopper 2 110 7 2 Chemistry Question Up201711171822 1510923166 8286UMANo ratings yet
- Chemistry IMU CET PDFDocument64 pagesChemistry IMU CET PDFAniket KNo ratings yet
- Alphonsa School, Kalamjote - Preboard - ChemistryDocument4 pagesAlphonsa School, Kalamjote - Preboard - Chemistryakshayashivakumar96No ratings yet
- ElecrochemistryDocument7 pagesElecrochemistryffxfuddiNo ratings yet
- Chemistry - 9 Time: 2 Hours M.M. 80: Section I (40 Marks) Attempt All Questions From This SectionDocument4 pagesChemistry - 9 Time: 2 Hours M.M. 80: Section I (40 Marks) Attempt All Questions From This SectionGreatAkbar1No ratings yet
- Https Doc 0c 0c Apps Viewer - GoogleusercontentDocument9 pagesHttps Doc 0c 0c Apps Viewer - GoogleusercontentAhmad RezaNo ratings yet
- Chapter 3 Electrochemistry Topic ElectrochemistryDocument16 pagesChapter 3 Electrochemistry Topic Electrochemistryvivek daveNo ratings yet
- 2003 Csec Chem Paper 01Document10 pages2003 Csec Chem Paper 01Jesshaun Morris100% (6)
- Karnataka Icse Schools Association: SECTION A (40 Marks)Document6 pagesKarnataka Icse Schools Association: SECTION A (40 Marks)Arebal100% (1)
- Eje Islamic f4 22 Chem 1-1Document7 pagesEje Islamic f4 22 Chem 1-1Nassrah JumaNo ratings yet
- Delhi Public School Newtown SESSION: 2021-22 Final Term Examination Class: Ix Total Marks: 80 Subject: Chemistry Time: 2 HoursDocument7 pagesDelhi Public School Newtown SESSION: 2021-22 Final Term Examination Class: Ix Total Marks: 80 Subject: Chemistry Time: 2 HoursSAMPURNA GHOSHNo ratings yet
- ElectrolysisDocument6 pagesElectrolysisskylar chanNo ratings yet
- Test - XII - 21.11.2023 - D & F Block Elements & OrganicDocument6 pagesTest - XII - 21.11.2023 - D & F Block Elements & Organicsaanvi2629jindalNo ratings yet
- IMUCET PCM CombinedDocument193 pagesIMUCET PCM Combinedshuklaity01No ratings yet
- Definition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-HomogeneousDocument8 pagesDefinition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-Homogeneousabdulrehman999No ratings yet
- Mit18 06scf11 Ses2.5sumDocument4 pagesMit18 06scf11 Ses2.5sumabdulrehman999No ratings yet
- Pureit Excella User Manual PDFDocument31 pagesPureit Excella User Manual PDFabdulrehman999No ratings yet
- Social ScienceDocument4 pagesSocial Scienceabdulrehman999No ratings yet
- Practical 12 Object: Tasks: Working With Arrays in C++Document6 pagesPractical 12 Object: Tasks: Working With Arrays in C++abdulrehman999No ratings yet
- Step-by-Step Calculator: Cosx Sin XDocument4 pagesStep-by-Step Calculator: Cosx Sin Xabdulrehman999No ratings yet
- Practical-8 To 10Document18 pagesPractical-8 To 10abdulrehman999No ratings yet
- Practical # 07 (16EL84)Document9 pagesPractical # 07 (16EL84)abdulrehman999No ratings yet
- English Grammar - LearnEnglish - British Council - Sentence StructureDocument12 pagesEnglish Grammar - LearnEnglish - British Council - Sentence Structureabdulrehman999No ratings yet
- Cost Accounting Mcqs - PaperpkinfoDocument8 pagesCost Accounting Mcqs - Paperpkinfoabdulrehman999100% (2)
- Chemistry SPM Potential Questions-Form5chap1 2Document15 pagesChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNo ratings yet
- Benzoic Acid From TolueneDocument66 pagesBenzoic Acid From TolueneIgnacio Real BuffelliNo ratings yet
- Canadian Business English Canadian 7th Edition Guffey Solutions ManualDocument35 pagesCanadian Business English Canadian 7th Edition Guffey Solutions Manualpeanutsofteniscd1n100% (31)
- Crude OilDocument8 pagesCrude OilAathifa ThowfeekNo ratings yet
- Chem (Final)Document17 pagesChem (Final)Jaynie Lee VillaranNo ratings yet
- Wet EtchingDocument15 pagesWet Etchingnskprasad89No ratings yet
- Organic Distinguishing Tests NotesDocument1 pageOrganic Distinguishing Tests Notesihshan007No ratings yet
- Baker Safe-T-DataDocument1 pageBaker Safe-T-DataJonathan Saviñon de los SantosNo ratings yet
- Preparation and Reactions of Boric Acid, H3BO3Document8 pagesPreparation and Reactions of Boric Acid, H3BO3Sin YeeNo ratings yet
- Product Catalogue 2011 12Document184 pagesProduct Catalogue 2011 12Jagesh RanjanNo ratings yet
- The Boron FamilyDocument27 pagesThe Boron Familygautambadgujar30No ratings yet
- Core (STEM) - EarthScience-SLMG11Q1W1-Identify Common Rock-Forming Minerals Using Their Physcial and Chemical PropertiesDocument18 pagesCore (STEM) - EarthScience-SLMG11Q1W1-Identify Common Rock-Forming Minerals Using Their Physcial and Chemical PropertiesRM L. JamandronNo ratings yet
- Class 12 Chemistry Set 1Document15 pagesClass 12 Chemistry Set 1latestdaaNo ratings yet
- Latihan NMR Dan FT-IRDocument37 pagesLatihan NMR Dan FT-IRWidi KurniaNo ratings yet
- Syllabus IOC I Pharm.D (C.L Baid)Document4 pagesSyllabus IOC I Pharm.D (C.L Baid)giridharan rajendranNo ratings yet
- Preservative Selection GuideDocument14 pagesPreservative Selection Guidedecker.19369No ratings yet
- TitrationDocument23 pagesTitrationAKSHAY MISHRA100% (1)
- Chapter 5 ConclusionDocument4 pagesChapter 5 ConclusionRaffandi RolandoNo ratings yet
- CHE 321 - CH5 - Fatty AlcoholDocument27 pagesCHE 321 - CH5 - Fatty AlcoholnorazifahNo ratings yet
- ACGDocument10 pagesACGrishichauhan25No ratings yet
- F-720-032 Rev. B O-Ring KitsDocument2 pagesF-720-032 Rev. B O-Ring KitsRD Rental CampoNo ratings yet
- Complejos de Cobalto ArtDocument3 pagesComplejos de Cobalto ArtNatalia MayaNo ratings yet
- Reaction of AlkynesDocument25 pagesReaction of AlkynesGabrielaAmandaNo ratings yet
- 50% Caustic Soda Membrane West Coast: Sales SpecificationDocument1 page50% Caustic Soda Membrane West Coast: Sales SpecificationMeziane BouktitNo ratings yet
- PTA Technology - FundamentalsDocument50 pagesPTA Technology - FundamentalsSarat Na NiNo ratings yet
- Class 12 Book 6 Organic Chemistry BiomoleculesDocument17 pagesClass 12 Book 6 Organic Chemistry Biomoleculesfakeu foreverNo ratings yet
- PPDDocument79 pagesPPDEddie ChangNo ratings yet
- Structured Questions: HKDSE Chemistry A Modern View Part X Chemical EquilibriumDocument26 pagesStructured Questions: HKDSE Chemistry A Modern View Part X Chemical EquilibriumNg Swee Loong StevenNo ratings yet
- CrystalDocument2 pagesCrystalAduchelab AdamsonuniversityNo ratings yet
- Optibor: Product Data SheetDocument5 pagesOptibor: Product Data Sheetanon_993394650No ratings yet