Professional Documents
Culture Documents
Shell & Tube Condenser Design: O O O O O O
Shell & Tube Condenser Design: O O O O O O
Uploaded by
tpchoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Shell & Tube Condenser Design: O O O O O O
Shell & Tube Condenser Design: O O O O O O
Uploaded by
tpchoCopyright:
Available Formats
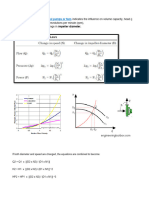
SHELL & TUBE CONDENSER DESIGN
NOTE:
DO NOT CHANGE THE FIGURES WHICH ARE COLOURED
STEP - 1
TO CALCULATE HEAT LOAD
1) SHELL SIDE FLUID (vapourizing fluid)
2) TUBE SIDE FLUID (utility)
INPUT DATA :
1) Cp OF SHELL SIDE FLUID HOT FLUID( Kcal/Kg OC)
2) Cp OF TUBE SIDE FLUID COLD FLUID( Kcal/Kg OC)
5) TEMP. OF HOT FLUID INLET ( OC )
6) TEMP. OF HOT FLUID OUTLET ( OC )
7) TEMP. OF COLD FLUID INLET ( OC )
8) TEMP. OF COLD FLUID OUTLET ( OC )
9)SP.GR. OF SHELL SIDE FLUID
10) LATENT HEAT OF VAPORISATION (Kcal/KG C)
11) FLOWRATE OF SHELL SIDE FLUID (Kg/Hr)
12) FLOWRATE OF TUBE SIDE UTILITY FLUID (Kg/Hr)
13) FLOWRATE OF TUBE SIDE UTILITY FLUID (m3/Hr)
STEP - 2
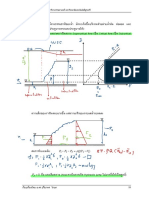
TO CALCULATE LMTD FOR COUNTER CURRENT FLOW :
HOT FLUID IN
41
COLD FLUID OUT
35
DIFFERENCE
6
HOT FLUID OUT
COLD FLUID IN
DIFFERENCE
STEP - 3
OVERALL HEAT TRANFER COEFFICIENT CONSIDERATION.
BTU/HrM2OK
CONSIDERED=
50
244
Kcal/M2.hr.C
STEP - 4
HEAT TRANSFER AREA CALCULATION
INPUT DATA
1) HEAT LOAD
2) LMTD
3) OVERALL HEAT TRANSFER COEFFICIENT
Heat Transfer Area ( M2 )
STEP - 5
AREA OF THE CONDENSER REQUIRED
(CONSIDERING 20 % DIVERSITY)
NSER DESIGN
water
water
1
0.35
41
35
30
35
1.32
87
792
42089
42
105.8 F
95 F
86 F
95 F
COLD FLUID
Total Boile up
Kg/Hr
m3/Hr
35
30
5
73656 Kcal/Hr
5.4848149
2 O
244 Kcal/Hr-M - C
55.037199
M2
66.04
M2
Acetonitrile
81.75
-47
Benzene
80.1
Carbon Bisulfide
46.3
Chloroform
Cyclohexane
DMF
152.8
EDC
600 torr
-59.4
500 torr
1.7
56.5
400 torr
139.6
Acetone
200 torr
Acetic Anhydride
100 torr
-17.2
63
80
99
105
110
82.2
100
119.8
126
133
7.7
22.7
39.5
46
51
15.9
27
43.7
62.5
68
74
7.6
15.4
26.1
42.2
60.6
67
73
-43.94
-21.16
-13.6
-5.1
10.4
28
34.57
39.5
-39.1
-29.7
-7.1
0.5
10.4
25.9
42.7
48
54
-25.4
-15.9
6.7
14.7
25.5
42
60.8
68
73
19.3
32.47
62.85
72.91
86.48
106.7
129.3
137.2
143.83
60 torr
118.1
40 torr
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
Solvent
10 torr
Acetic Acid
Sr.N
o.
5 torr
1 torr
Common Solvent Data Sheet
B.P. in
o
C at
Atm Pr.
6.3
17.5
43
51.7
24.8
36
62.1
70.8
-40.5
-31.1
-9.4
-2
-26.6
-16.3
7.7
-36.7
-19.6
-11.5
-73.87
-53.8
61.3
-58
80.7
-45.3
84
-44.5
-24
-13.6
10
18.1
29.4
45.7
64
70
76
Ethanol
78.4
-31.3
-12
-2.3
19
26
34.9
48.4
63.5
68
72
EPH
117.9
-16.5
5.6
16.6
42
50.6
62
79.3
98
Ethyl Acetate
77
-43.4
-23.5
-13.5
9.1
16.6
27
42
59.3
64
70
FFA
146
14.5
27.59
57.48
67.38
80.74
100.62
124.88
130.62
137.17
Formaldehyde
-19.5
-88
-70.6
-65
-57.3
-46
-33
-32
-27
IPA
82.5
-26.1
-7
2.4
23.8
30.5
39.5
53
67.8
70
77
MDC
39.75
-70
-52.1
-43.3
-22.3
-15.7
-6.3
24.1
30
34
Methanol
64.7
-44
-25.3
-16.2
12.1
21.2
34.8
49.9
55
59
33
-82.17
-62.99
-53.49
-31.65
-24.42
-14.66
-0.14
16.11
21.76
26.55
MIPA
Mono Chloro Benzene
132
-13
10.6
22.2
49.7
58.3
70.7
89.4
110
117
123
MTBE
55.2
-68.32
-47.75
-37.56
-14.14
-6.38
4.07
19.65
37
43.15
48.28
n-Butanol
117.5
-1.2
n-Hexane
68.7
Petroleum Ether
60-80
20
30.2
41.5
60.3
70.1
84.3
100.8
106
111
-34.5
-25
-2.3
5.4
15.8
31.6
49.6
56
61
172
Phenol
182
40.1
62.5
73.8
100.1
108.4
121.4
139
160
168
Pyridine
115.4
-18.9
2.5
13.2
38
46.8
57.8
75
95.6
108
108
66
-61.58
-40.34
-29.81
-5.62
2.39
13.19
29.28
47.29
53.55
58.85
110.6
-26.7
-4.4
6.4
31.8
40.3
51.9
69.5
89.5
97
102
1.2
11.2
34
41.5
51.6
66.5
79.41
86.3
92.13
THF
Toluene
Water
100
o-Xylene
139.3
-15.86
9.97
22.78
52.2
61.94
75.08
94.65
116.55
124.16
130.61
m- Xylene
144
-12.93
13.2
26.15
55.9
65.76
79
98.84
120.99
128.69
135.21
p- Xyleme
137-138
Aniline
184
35
58
69.4
97
106
119
140
162
Carbon t Chloride
76.7
-50
-30
-20
4.3
12.3
23
38.3
57.8
Di Ethyl Ether
34.6
-74
-57
-48
-28
-22
-12
2.2
17.9
Dimethyl Sulfoxide
189
33
60
73
103
114
125
146
178
Dioxane
101
-36
-13
-1.2
25
33.8
45.1
62.3
81.8
Ethelene Glycol
197
53
78
92
120
130
142
159
179
Formamide
211
71
96
110
138
147
158
176
194
Methyl Acetate
57.8
-57
-39
-29
-8
-0.5
9.4
24
40
NitroBenzene
211
44
72
84.9
115
129
140
161
186
Propanol -1
82
97.8
-15
14.7
36
43.5
52.8
66.8
Heptane
98
-34
-13
-2
22
31
42
59
78
Glycerine
290
126
154
167
198
208
220
240
263
700 torr
Latent
Heat (Cal
Sp.Gr.
/gm)
115
1.053
96.75
138
1.082
55
0.78
79
78
43.9
Sp. Heat
Cp
(Kcal/Kgo
C)
Flash Point Viscosity
Vap.
Melting
(Close Cup) in CP (25 Density point
o
(oC)
C)
(Kg/m3)
(oC)
0.522
39
1.15
2.07
96.44
0.236
48.8
0.91
124.3
0.538
-18
0.33
0.783
173.68
0.541
0.38
0.8787
103
0.419
-11
0.65
1.263
59.35
-30
NA
59
1.484
59.01
0.231
none
0.57
78.5
0.7781
93.81
0.44
-18
0.98
149.6
0.945
137.81
0.486
58
0.82
82
1.2569
77.33
0.3015
13
0.9
3.35
76
0.789
204.26
0.68
13
1.2
1.62
1.183
97.95
94.26
0.457
-4.4
0.46
Mol.
Wt.
Solubility
in water at
20 oC (%
w/w)
16.7
60.05
3.52
-73
102.09
12
-94.6
58.08
infinite
1.5
0.41
41.05
infinite
5.5
78.11
0.18
-108.6
76.13
4.12
-63.5
119.4
0.82
2.9
6.6
54.16
0.0052
-61
73.1
infinite
-40
98
NIL
-112
46.07
infinite
-25.6
92.53
-82.4
88.1
3.05
infinite
74
0.901
142.87
1.03
7.7
-24
0.815
-92
30.03
Very soluble
80
0.785
159.35
0.67
-11.7
0.34
2.07
-101
60.1
infinite
37.5
1.3255
77.077
0.2888
None
0.44
2.93
-96.7
84.94
63
0.7915
269.79
0.605
12
..65
1.1
-97.8
32.04
infinite
30.72
0.692
119.09
0.65
129
1.107
78.3
0.311
29
0.8
52.75
0.747
78.196
0.508
-28
0.35
114
0.81
141.26
0.687
35
294
66
0.6591
80.48
0.527
-18
0.31
infinite
60
0.635
0.498
-40
59.111
3.04
2.97
-45.2
112.56
-109
88.15
0.049
4.8
-79.9
74.12
7.45
-94
86.17
0.001
2.5
178
1.071
0.561
79
2.8
113
0.982
107.36
40.6
94.11
8.2
0.431
20
0.88
-42
79.1
infinite
63.47
0.889
92.414
107
0.866
88.17
0.325
4.4
0.59
97.22
0.99
540
0.99
NA
0.89
136.22
0.88
81.86
0.4
27
0.85
140.89
0.87
81.86
0.4
32
0.86
81.86
0.4
1.032
103
0.512
46
0.2
0.713
123
0.473
-40
0.24
74.12
6.9
1.101
178
0.7
85
1.99
78
Fully Miscible
1.034
102
0.4
12
1.3
88
Fully Miscible
1.113
191
0.573
>110
20.9
62
Fully Miscible
1.134
112
0.595
154
3.8
45.04
Fully Miscible
0.932
98
0.4
-9
0.37
-98.7
74.08
24.5
1.196
79
0.339
87
1.86
5.7
123.11
0.19
0.804
164
0.582
15
1.72
60.1
Fully Miscible
0.684
87.18
0.518
-1
0.41
1.261
124.17
0.4
160
945
-17.22
2.5
-108
72.1
Fully Miscible
3.14
-95
92.13
0.06
18
100
3.6
-47.4
106.1
NIL
0.6
3.6
-25
106.4
0.02
27
0.65
3.6
13-14
106.1
NIL
70
3.8
-6.2
93.13
3.8
-22.6
153.84
9.5-10.5
9.6
100.21
0.005
92.09
Fully Miscible
Acetone Condenser
Tray Oven
Acetone Liquid
Coolant Fluid Out
Heat Input
Basis of Drying Oven Operation:
1.
2.
3.
4.
Wet solid product is put into Tray Oven; oven is sealed closed.
Heat input is applied to oven;
Cooling fluid is circulated through acetone condenser;
vacuum pump is started;
As the wet solid is heated in the oven, the vapor pressure of the acetone increases and the acetone starts to vaporiz
produced by the vacuum pump;
The vacuum pump works to maintain the set partial vacuum that is controlled by allowing atmospheric air to "bleed" i
vacuum pump - under ideal conditions - would not not normally have to continue to operate after extracting all non-co
in the real world situation, there will be air infiltration into the partial vacuum system through the oven door seals, the
These air infiltrations try to defeat the vacuum created and, as a consequence, the vacuum pump is left to continue t
by allowing the atmospheric air to bleed in and keep the pump working at capacity.
The partial vacuum is really set by the temperature of the cooling fluid in the acetone condenser. The acetone has t
that corresponds to the acetone vapor pressure equal to the partial vacuum setting. This allows the acetone to cond
as a liquid and not be "sucked" (extracted) out of the system by the vacuum pump.
If the temperature of the condensed acetone is sufficiently cold, there will little or undetected losses of acetone exitin
The addition of heat into the oven accelerates the rate of acetone evaporation and speeds up the drying process; the
the cold energy that has to be inputted into the condenser as the coolant fluid.
Vacuum Pump
ant Fluid Out
acetone starts to vaporize at the lower, partial vacuum pressure
mospheric air to "bleed" into the vacuum pump suction. The
after extracting all non-condensables from the system. However,
the oven door seals, the gaskets, equipment and piping joints, etc., etc.
pump is left to continue to operate - although at a reduced capacity
nser. The acetone has to be cooled and condensed at a temperature
llows the acetone to condense and drop out of the condenser
d losses of acetone exiting in the vacuum pump exhaust to atmosphere.
up the drying process; the price to pay for this capacity increase is
You might also like
- Sizing For Vaporiser/Flash Vessel: InputsDocument3 pagesSizing For Vaporiser/Flash Vessel: InputsSaravana ChandranNo ratings yet
- Chemical Compatibility GuideDocument41 pagesChemical Compatibility Guidetpcho100% (1)
- Chemical Compatibility GuideDocument41 pagesChemical Compatibility Guidetpcho100% (1)
- Ammonia Wet Scrubber System (17-7-2018)Document12 pagesAmmonia Wet Scrubber System (17-7-2018)addin wokatuba100% (1)
- Aspen Case 4 RDocument137 pagesAspen Case 4 RItxaso Villanueva OraaNo ratings yet
- Major Project PPT GGBSDocument20 pagesMajor Project PPT GGBSNaReN KumarNo ratings yet
- En - 13384 2 EnglishDocument61 pagesEn - 13384 2 Englishbadmike71No ratings yet
- Distillation Column: Major Equipment DesignDocument45 pagesDistillation Column: Major Equipment Designrubesh_rajaNo ratings yet
- Blowdown Vessel CalculationDocument1 pageBlowdown Vessel CalculationOmkarNo ratings yet
- Centrif Pumps1 SpreadsheetDocument2 pagesCentrif Pumps1 SpreadsheetCast Ed Iv0% (1)
- Tank Venting Capacity-Fire CaseDocument1 pageTank Venting Capacity-Fire CaseAjay TiwariNo ratings yet
- Stacks: Ammonia Injection: A Route To CleanDocument8 pagesStacks: Ammonia Injection: A Route To CleanZEN MA100% (1)
- Air Cooler Design (Ujian)Document36 pagesAir Cooler Design (Ujian)Wahyu JatiNo ratings yet
- Flash CalculationsDocument10 pagesFlash CalculationsHamza AliNo ratings yet
- Pipe DimensionDocument7 pagesPipe DimensionLily NurdianaNo ratings yet
- Project No. 16279S Project Name Bapco - JVTST: Calculation of Emissivity of Process GasDocument11 pagesProject No. 16279S Project Name Bapco - JVTST: Calculation of Emissivity of Process GasrajachemNo ratings yet
- Timah - Open Spray - Tower - For - Flue - Gas - Scrubbing - Design 56870 NCMHDocument1 pageTimah - Open Spray - Tower - For - Flue - Gas - Scrubbing - Design 56870 NCMHAyahKenzie100% (1)
- Flammability WorksheetDocument6 pagesFlammability WorksheetshailendraNo ratings yet
- Flue Gas 360 PDFDocument2 pagesFlue Gas 360 PDFwatson123No ratings yet
- Heatcalc: A Natural Gas Heat of Combustion CalculatorDocument7 pagesHeatcalc: A Natural Gas Heat of Combustion CalculatorMuzzamilNo ratings yet
- IncineratorDocument6 pagesIncineratorKamal DeshapriyaNo ratings yet
- Specification Sheet For Separator: Operating Conditions SketchDocument12 pagesSpecification Sheet For Separator: Operating Conditions SketchAngelikaOdimer100% (1)
- RD 810Document73 pagesRD 810Ashish MishraNo ratings yet
- Shell and Tube Heat Exchanger Design: Temperature of FluidsDocument6 pagesShell and Tube Heat Exchanger Design: Temperature of FluidsRashmi PariharNo ratings yet
- Fire Relief Dynamic StudyDocument2 pagesFire Relief Dynamic StudylguardiaNo ratings yet
- Hitungan PPDocument30 pagesHitungan PPHamdan ShdNo ratings yet
- Pump CalcDocument121 pagesPump Calcravirawat15No ratings yet
- Batch Time Calculation For Isothermal Cooling in Internal Coil Vessel With AgitationDocument2 pagesBatch Time Calculation For Isothermal Cooling in Internal Coil Vessel With Agitationdhavalesh1100% (1)
- N2 RequirementDocument2 pagesN2 RequirementsandeshNo ratings yet
- Ammonia Plant Simulation 25.08.2016Document81 pagesAmmonia Plant Simulation 25.08.2016Manish Gautam100% (1)
- SP SP VP V PR) : Velocity Pressure Method Calculation SheetDocument6 pagesSP SP VP V PR) : Velocity Pressure Method Calculation SheetAnkit LonareNo ratings yet
- Design of Distillation Columns - ImpDocument13 pagesDesign of Distillation Columns - ImpAlla VijayNo ratings yet
- REBOILER (RB-101) : Operating ConditionsDocument27 pagesREBOILER (RB-101) : Operating ConditionsBenedick Jayson MartiNo ratings yet
- Ogpe OGPE Oil Fields: Calculation SheetDocument4 pagesOgpe OGPE Oil Fields: Calculation SheetFaber TrujilloNo ratings yet
- Separator Sizing SpreadsheetDocument10 pagesSeparator Sizing SpreadsheetEmmanuel ByensitaNo ratings yet
- Cooling Water Line HydDocument7 pagesCooling Water Line HydpavanNo ratings yet
- Section 7 - Separation EquipmentDocument9 pagesSection 7 - Separation Equipmentlulis171No ratings yet
- Heat Conduction Pipe InsulationDocument1 pageHeat Conduction Pipe InsulationJogender DhayalNo ratings yet
- Orifice Sizing TemplateDocument2 pagesOrifice Sizing TemplaterajeevjayanathNo ratings yet
- Horizontal KO PotDocument4 pagesHorizontal KO Pothk168No ratings yet
- Heat Transfer. Exchangers Design. Effectivness and Number of Transfer Units NTUDocument70 pagesHeat Transfer. Exchangers Design. Effectivness and Number of Transfer Units NTUHenry WicaksanaNo ratings yet
- Packed Column Calculation Results: Packing Details System DetailsDocument1 pagePacked Column Calculation Results: Packing Details System Detailsdinakaranpatel100% (1)
- Relief Load CalculationDocument8 pagesRelief Load CalculationMuthuKumar ArunachalamNo ratings yet
- Vent Line Pressure Drop CalculationDocument4 pagesVent Line Pressure Drop CalculationRubensBoerngenNo ratings yet
- Operating Data : Two Pahse Horizental Separator Sizing From Total CoDocument3 pagesOperating Data : Two Pahse Horizental Separator Sizing From Total CoadammzjinNo ratings yet
- Vapor Line Sizing-Mpp6Document10 pagesVapor Line Sizing-Mpp6Nitin KurupNo ratings yet
- Pump Affinity (K0 SNW)Document6 pagesPump Affinity (K0 SNW)Myat Kyaw HeinNo ratings yet
- Heat Exchanger Area & Boilup Rate CalculationDocument19 pagesHeat Exchanger Area & Boilup Rate CalculationNitin KurupNo ratings yet
- See Sheet API-2000: Normal Venting Liquid Movement Thermal EffectDocument33 pagesSee Sheet API-2000: Normal Venting Liquid Movement Thermal EffecthhvgNo ratings yet
- Steam Ejector CalculationsDocument7 pagesSteam Ejector Calculationshoangvubui4632No ratings yet
- Matreail BalanceDocument1 pageMatreail BalanceATUL SONAWANENo ratings yet
- Chimney Draught Calculations: //vboxsrv/conversion - Tmp/scratch - 4/195995390.xls - Ms - OfficeDocument1 pageChimney Draught Calculations: //vboxsrv/conversion - Tmp/scratch - 4/195995390.xls - Ms - OfficemohdnazirNo ratings yet
- Mass Balance (Final)Document26 pagesMass Balance (Final)Adeel AhmedNo ratings yet
- Condensate Line SizingDocument4 pagesCondensate Line SizingMubin Ashraf SheikhNo ratings yet
- 20120507091359 (1)Document6 pages20120507091359 (1)Noman Abu-FarhaNo ratings yet
- XSteam Excel v2.6Document3 pagesXSteam Excel v2.6pchanycNo ratings yet
- Cooling Tower. Merkel Theory - TreybalDocument39 pagesCooling Tower. Merkel Theory - TreybalMarx CesarNo ratings yet
- Bell Method Example 7 5Document9 pagesBell Method Example 7 5jnmanivannanNo ratings yet
- Upl HBDSDocument32 pagesUpl HBDSSuparna BhoseNo ratings yet
- Tutorial Steam GenerationDocument2 pagesTutorial Steam GenerationAmanda KodippiliNo ratings yet
- Bkf4143-Process Engineering Economics 11213 PDFDocument11 pagesBkf4143-Process Engineering Economics 11213 PDFJeevanNairNo ratings yet
- Water Chillers R407C - R22: Air Cooled Water Chillers With Scroll Compressors and Axial FansDocument4 pagesWater Chillers R407C - R22: Air Cooled Water Chillers With Scroll Compressors and Axial FansgoodtiNo ratings yet
- Prediction of Reaction Enthalpy and Adiabatic Temperature RiseDocument20 pagesPrediction of Reaction Enthalpy and Adiabatic Temperature RisetpchoNo ratings yet
- Itrform12bb PDFDocument3 pagesItrform12bb PDFtpchoNo ratings yet
- Petrofac WebinarDocument6 pagesPetrofac WebinarrrusNo ratings yet
- Dry HCL Gas HandlingDocument5 pagesDry HCL Gas HandlingtpchoNo ratings yet
- HydrogenationDocument3 pagesHydrogenationtpchoNo ratings yet
- Pfaudler Mixing SystemsDocument6 pagesPfaudler Mixing SystemstpchoNo ratings yet
- JS Pressure Safety Valve FinalDocument28 pagesJS Pressure Safety Valve FinaltpchoNo ratings yet
- Disclosure To Promote The Right To InformationDocument9 pagesDisclosure To Promote The Right To InformationtpchoNo ratings yet
- Evaporators PDFDocument31 pagesEvaporators PDFtpcho100% (5)
- Reboilers PDFDocument0 pagesReboilers PDFtpchoNo ratings yet
- Distillation Column Design: Syed Zaheer AbbasDocument21 pagesDistillation Column Design: Syed Zaheer AbbastpchoNo ratings yet
- Honeywell-Sensing-Kgz10 Series Oxygen SensorsDocument4 pagesHoneywell-Sensing-Kgz10 Series Oxygen SensorstpchoNo ratings yet
- CH 5 Ion PracticeDocument2 pagesCH 5 Ion PracticeMahmoud AladdasiNo ratings yet
- Instrukcja EasyMIG210 210s 215 225 EN-1Document28 pagesInstrukcja EasyMIG210 210s 215 225 EN-1Nick INo ratings yet
- Summer Training ReportDocument24 pagesSummer Training ReportJayant MathurNo ratings yet
- General Chemistry 1: Quarter 1 - Module 3 Determining Molar Mass Chemical Reactions and EquationsDocument26 pagesGeneral Chemistry 1: Quarter 1 - Module 3 Determining Molar Mass Chemical Reactions and EquationsVima SanchezNo ratings yet
- Variable Head Type FlowmetersDocument32 pagesVariable Head Type FlowmetersAnuNarayan R100% (2)
- A11.18 - Mobilux EP 2Document1 pageA11.18 - Mobilux EP 2Dony LieNo ratings yet
- SMS - Thin Film Evaporator PDFDocument20 pagesSMS - Thin Film Evaporator PDFdj22500100% (1)
- AnswersDocument5 pagesAnswers22 shantanu kapadnisNo ratings yet
- Me688 UsmDocument39 pagesMe688 UsmKETU PRINCE LEKUNo ratings yet
- Introduction To Environmental EngineeringDocument12 pagesIntroduction To Environmental EngineeringTemesgen SemagnNo ratings yet
- XI Science PDFDocument26 pagesXI Science PDFCreative GamingNo ratings yet
- Gas Turbine CoolingDocument13 pagesGas Turbine CoolingHimanshu DahireNo ratings yet
- Bengkel Biologi SmartGDocument6 pagesBengkel Biologi SmartGK XuanNo ratings yet
- Corrosion and Its PreventionDocument28 pagesCorrosion and Its PreventionMehul Neha Bisht100% (2)
- Gold Ore Processing PlantDocument4 pagesGold Ore Processing Plantjafer2000No ratings yet
- Petroleum Chemicals OverviewDocument1 pagePetroleum Chemicals OverviewhamburgermcgintyNo ratings yet
- Chapter 1 Solutions DetailedDocument30 pagesChapter 1 Solutions DetailedYeonjae JeongNo ratings yet
- 2017mar11 PHY1001 Assignment 4Document2 pages2017mar11 PHY1001 Assignment 4AbhimanyuNo ratings yet
- Acoustic Insulation Under FloorDocument11 pagesAcoustic Insulation Under FloorMiteshaNo ratings yet
- Mining: Supercritical Flow Critical Flow Subcritical FlowDocument3 pagesMining: Supercritical Flow Critical Flow Subcritical FlowBoonsita NammanaNo ratings yet
- Piu RajakDocument18 pagesPiu Rajakpiu_rajakNo ratings yet
- The Real Trick To Drying Ceramic Ware Is To Use A Method That Removes The Water From The Inside of The Ceramic As Fast As The Surface Water Is EvaporatedDocument55 pagesThe Real Trick To Drying Ceramic Ware Is To Use A Method That Removes The Water From The Inside of The Ceramic As Fast As The Surface Water Is EvaporatedMiguel OcampoNo ratings yet
- Edxrf VS WDXRF PDFDocument5 pagesEdxrf VS WDXRF PDFkulihat_hijauNo ratings yet
- Innovations in The Food Packaging Market - Intelligent Packaging - A ReviewDocument6 pagesInnovations in The Food Packaging Market - Intelligent Packaging - A ReviewISABELLA CUENÚNo ratings yet
- Impreglon 222M DataSheetDocument3 pagesImpreglon 222M DataSheetKyle S JonesNo ratings yet
- PH537/PI515: Astrostructure and Evolution: Answer With Examples and ReferencesDocument4 pagesPH537/PI515: Astrostructure and Evolution: Answer With Examples and ReferencesSDasNo ratings yet
- MSDS New BERKOCLEAN 508Document5 pagesMSDS New BERKOCLEAN 508haiderparrai00No ratings yet