Professional Documents
Culture Documents
Tetralogy of Fallot
Tetralogy of Fallot
Uploaded by
Laurice BarronCopyright:
Available Formats
You might also like
- Medicine in Brief: Name the Disease in Haiku, Tanka and ArtFrom EverandMedicine in Brief: Name the Disease in Haiku, Tanka and ArtRating: 5 out of 5 stars5/5 (1)
- Tetralogy of FallotDocument28 pagesTetralogy of FallotArdyansyah Nasution, M.DNo ratings yet
- Tetralogy of Fallot Lesson PlanDocument25 pagesTetralogy of Fallot Lesson PlanUday Kumar100% (1)
- Sample Process Recording ColumnDocument6 pagesSample Process Recording ColumnLaurice Barron100% (1)
- Tetralogy of FallotDocument28 pagesTetralogy of FallotconcozNo ratings yet
- Tetralogy of FallotDocument8 pagesTetralogy of Fallotdecembergirl92No ratings yet
- Tetralogy of FallotDocument8 pagesTetralogy of FallotHillary Faye FernandezNo ratings yet
- Tetralogy of FallotDocument7 pagesTetralogy of FallotAshok Kumar JangirNo ratings yet
- Tetralogy of Fallot.......Document7 pagesTetralogy of Fallot.......Sam AlipioNo ratings yet
- Tetralogy of FallotDocument65 pagesTetralogy of FallotEndrati WidiastutiNo ratings yet
- Background TOFDocument2 pagesBackground TOFNyitnyit KunyitNo ratings yet
- Tetralogy of FallotDocument9 pagesTetralogy of FallotPaul MagbooNo ratings yet
- What Is Tetralogy of FallotDocument3 pagesWhat Is Tetralogy of FallotJyedenn PonceNo ratings yet
- What Is Tetralogy of FallotDocument2 pagesWhat Is Tetralogy of FallotJamaica Cruz San Pedro100% (1)
- Tetralogy of FallotDocument5 pagesTetralogy of FallotCharity OaniaNo ratings yet
- TOFDocument17 pagesTOFMaryel PascualNo ratings yet
- What Is Tetralogy of Fallot?: Congenital Heart DefectDocument3 pagesWhat Is Tetralogy of Fallot?: Congenital Heart DefectGlenn Asuncion PagaduanNo ratings yet
- Tetralogy of Fallot Is A Combination of Four Congenital AbnormalitiesDocument4 pagesTetralogy of Fallot Is A Combination of Four Congenital AbnormalitiesJoanne TolopiaNo ratings yet
- Tetralogy of Fallot: (A Congenital Heart Disease)Document7 pagesTetralogy of Fallot: (A Congenital Heart Disease)Sunil Kumar SahuNo ratings yet
- Tetralogy of FallotDocument7 pagesTetralogy of FallotWidelmark FarrelNo ratings yet
- Tetralogy of FallotDocument3 pagesTetralogy of FallotMiLody NajeNo ratings yet
- Tetralogy of Fallot: Arianna Jasminemabunga Bsn-2BDocument30 pagesTetralogy of Fallot: Arianna Jasminemabunga Bsn-2BArianna Jasmine MabungaNo ratings yet
- Tetralogy of Fallot - StatPearls - NCBI BookshelfDocument9 pagesTetralogy of Fallot - StatPearls - NCBI BookshelfElfrida AuliaNo ratings yet
- Magyawi Tetralogy of Fallot 1st PartDocument7 pagesMagyawi Tetralogy of Fallot 1st PartJoanna Bee Rose MagyawiNo ratings yet
- Tetralogy of FallotDocument33 pagesTetralogy of FallotjeenaejyNo ratings yet
- Tetralogy of FallotDocument6 pagesTetralogy of FallotArianna Jasmine MabungaNo ratings yet
- Tetralogy of Fallot SP B.inggrisDocument19 pagesTetralogy of Fallot SP B.inggrisSoraya VerinaNo ratings yet
- Presentation ON Tetrallogy of FallotDocument33 pagesPresentation ON Tetrallogy of Fallotvani reddyNo ratings yet
- Tetralogy of FallotDocument38 pagesTetralogy of FallotJohn Paul MedalloNo ratings yet
- Congenital AnomaliesDocument26 pagesCongenital AnomaliesMaria VisitacionNo ratings yet
- Congenital Heart Defec1Document3 pagesCongenital Heart Defec1Matt BaterzalNo ratings yet
- Cyanotic Spells Blue Spells in Tetralogy of Fallot - PIAG 214Document2 pagesCyanotic Spells Blue Spells in Tetralogy of Fallot - PIAG 214imil irsalNo ratings yet
- Tof Case ReportDocument11 pagesTof Case ReportPatrick DycocoNo ratings yet
- LESSON 3. Congenital Heart DefectsDocument9 pagesLESSON 3. Congenital Heart DefectsEdmarie P. TabacoldeNo ratings yet
- Odia Tetralogy of FallotDocument25 pagesOdia Tetralogy of Fallotvictorjonathan567No ratings yet
- Presented by DR Rahul D AgrawalDocument64 pagesPresented by DR Rahul D AgrawalRahul AgrawalNo ratings yet
- Congenital Heart DefectDocument2 pagesCongenital Heart DefectMatt BaterzalNo ratings yet
- Week 11 Post Test - Tof Group 29 12CDocument12 pagesWeek 11 Post Test - Tof Group 29 12CShane LuyNo ratings yet
- Tetralogy of FallotDocument31 pagesTetralogy of FallotDevipriya MajumderNo ratings yet
- Tetralogy of Fallot: Clinical Background AnatomyDocument12 pagesTetralogy of Fallot: Clinical Background AnatomyJoriza TamayoNo ratings yet
- Tetralogy of Fallot, Agarwala 2017Document5 pagesTetralogy of Fallot, Agarwala 2017rinayondaNo ratings yet
- Congenital Heart Diseases.Document86 pagesCongenital Heart Diseases.Salman KhanNo ratings yet
- Presentation ON Tetralogy of FallotDocument36 pagesPresentation ON Tetralogy of Fallotvani reddyNo ratings yet
- Tetralogy of FallotDocument31 pagesTetralogy of FallotAnditha Namira RS100% (1)
- Fallot TetralogyDocument20 pagesFallot Tetralogykgt88No ratings yet
- What Is Tetralogy of FallotDocument18 pagesWhat Is Tetralogy of FallotIyah Bu-ucanNo ratings yet
- Tetralogy of FallotDocument5 pagesTetralogy of FallotNoor FadhilaNo ratings yet
- Tetralogy of Fallot Report 1Document9 pagesTetralogy of Fallot Report 1Maricar EustaquioNo ratings yet
- Cardiovascular System Dr. Eman Badr 2020Document182 pagesCardiovascular System Dr. Eman Badr 2020Amina DinarNo ratings yet
- 12 - Semiotics of Cardiovascular Disorders. Semiotics of Congenital Hert Diseases in Children.Document30 pages12 - Semiotics of Cardiovascular Disorders. Semiotics of Congenital Hert Diseases in Children.Omowunmi KadriNo ratings yet
- Tetralogy of FallotDocument3 pagesTetralogy of FallotyasminNo ratings yet
- Pediatric Patent Ductus ArteriosusDocument12 pagesPediatric Patent Ductus Arteriosusabirami_murugesuNo ratings yet
- Tetralogy of FallotDocument5 pagesTetralogy of FallotSaloni MehtaNo ratings yet
- Tetralogy of Fallot: What Is It?Document3 pagesTetralogy of Fallot: What Is It?Anditha Namira RSNo ratings yet
- Pulmonary Stenosis: Narrowing of The Pulmonary Valve and Outflow Tract or Area BelowDocument1 pagePulmonary Stenosis: Narrowing of The Pulmonary Valve and Outflow Tract or Area BelowGlenn Asuncion PagaduanNo ratings yet
- Tetralogy of FallotDocument12 pagesTetralogy of Fallotneetuthakur100% (5)
- Tetralogy of FallotDocument10 pagesTetralogy of FallotanggiehardiyantiNo ratings yet
- Tiki Taka Notes Final PDFDocument104 pagesTiki Taka Notes Final PDFAditiSahak62No ratings yet
- Congenital Heart Diseases, A Simple Guide to these Medical ConditionsFrom EverandCongenital Heart Diseases, A Simple Guide to these Medical ConditionsNo ratings yet
- Csa SampleDocument12 pagesCsa SampleLaurice BarronNo ratings yet
- Colonoscopy Is Mandatory AfterDocument3 pagesColonoscopy Is Mandatory AfterLaurice BarronNo ratings yet
- Corruption in The Military Audited by Heidi MendozaDocument1 pageCorruption in The Military Audited by Heidi MendozaLaurice BarronNo ratings yet
Tetralogy of Fallot
Tetralogy of Fallot
Uploaded by
Laurice BarronOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tetralogy of Fallot
Tetralogy of Fallot
Uploaded by
Laurice BarronCopyright:
Available Formats
TETRALOGY OF FALLOT
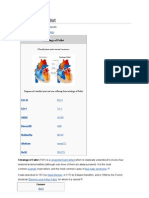
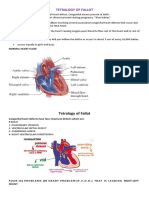
BACKGROUND HISTORY Tetralogy of Fallot (TOF) (teh-tral-uh-je ov fuh-LOE) is one of the most common congenital heart disorders (CHDs). This condition is classified as a cyanotic heart disorder, because tetralogy of Fallot results in an inadequate flow of blood to the lungs for oxygenation (right-toleft shunt) (see the following image). Patients with tetralogy of Fallot initially present with cyanosis shortly after birth, thereby attracting early medical attention. Historical information Louis Arthur Fallot, after whom the name tetralogy of Fallot is derived, was not the first person to recognize the condition. Stensen first described it in 1672; however, it was Fallot who first accurately described the clinical and complete pathologic features of the defects. Although the disorder was clinically diagnosed much earlier, no treatment was available until the 1940s. Cardiologist Helen Taussig recognized that cyanosis progressed and inevitably led to death in infants with tetralogy of Fallot. She postulated that the cyanosis was due to inadequate pulmonary blood flow. Her collaboration with Alfred Blalock led to the first type of palliation for these infants. In 1944, Blalock operated on an infant with tetralogy of Fallot and created the first Blalock-Taussig shunt between the subclavian artery and the pulmonary artery. Incidence Prenatal factors associated with a higher incidence of tetralogy of Fallot (TOF) include: maternal rubella (or other viral illnesses) during pregnancy, poor prenatal nutrition, maternal alcohol use, maternal age older than 40 years, maternal phenylketonuria (PKU) birth defects, and diabetes. Children with Down syndrome also have a higher incidence of tetralogy of Fallot, as do infants with fetal hydantoin syndrome or fetal carbamazepine syndrome. Tetralogy of Fallot is a rare condition that affects approximately 5 out of every 10,000 newborns. Characteristics of Tetralogy of Fallot Tetralogy of Fallot involves four heart defects: A large ventricular septal defect (VSD) Pulmonary (PULL-mun-ary) stenosis Right ventricular hypertrophy (hi-PER-tro-fe) An overriding aorta
Ventricular Septal Defect The heart has an inner wall that separates the two chambers on its left side from the two chambers on its right side. This wall is called a septum. The septum prevents blood from mixing between the two sides of the heart. A VSD is a hole in the septum between the heart's two lower chambers, the ventricles. The hole allows oxygen-rich blood from the left ventricle to mix with oxygen-poor blood from the right ventricle. Overriding Aorta This defect occurs in the aorta, the main artery that carries oxygen-rich blood from the heart to the body. In a healthy heart, the aorta is attached to the left ventricle. This allows only oxygenrich blood to flow to the body. In tetralogy of Fallot, the aorta is located between the left and right ventricles, directly over the VSD. As a result, oxygen-poor blood from the right ventricle flows directly into the aorta instead of into the pulmonary artery. Right Ventricular Hypertrophy With this defect, the muscle of the right ventricle is thicker than usual. This occurs because the heart has to work harder than normal to move blood through the narrowed pulmonary valve. Pulmonary Stenosis This defect involves narrowing of the pulmonary valve and the passage from the right ventricle to the pulmonary artery. Normally, oxygen-poor blood from the right ventricle flows through the pulmonary valve and into the pulmonary artery. From there, the blood travels to the lungs to pick up oxygen. In pulmonary stenosis, the pulmonary valve cannot fully open. Thus, the heart has to work harder to pump blood through the valve. As a result, not enough blood reaches the lungs.
ANATOMY AND PHYSIOLOGY With tetralogy of Fallot, not enough blood is able to reach the lungs to get oxygen, and oxygenpoor blood flows to the body. Cross-Section of a Normal Heart and a Heart With Tetralogy of Fallot
Figure A shows the structure and blood flow inside a normal heart. Figure B shows a heart with the four defects of tetralogy of Fallot. Babies and children who have tetralogy of Fallot have episodes of cyanosis (si-ah-NO-sis). Cyanosis is a bluish tint to the skin, lips, and fingernails. It occurs because the oxygen level in the blood leaving the heart is below normal. Tetralogy of Fallot is repaired with open-heart surgery, either soon after birth or later in infancy. The timing of the surgery will depend on how narrow the pulmonary artery is. Over the past few decades, the diagnosis and treatment of tetralogy of Fallot have greatly improved. Most children who have this heart defect survive to adulthood. However, they'll need lifelong medical care from specialists to help them stay as healthy as possible.
PATHOPHYSIOLOGY Environmental and genetic factor
Narrowed pulmonary valve and hole in the septum
Left (pumps oxygenated blood) and right (pumps unoxygenated blood) side of heart pumps the same blood as one causing blood shunts from right to left
Left side operated 4x higher than the right side as the right is weaker because of the aorta is attached to the right ventricle causing overriding aorta
The blood from the RV makes it difficult to travel blood to the pulmonary artery because of the narrowed pulmonary valve causing blood shunted to the LV via VSD
Results in the less blood going to the lungs to absorb oxygen and the deoxygenated blood mixing with the oxygenated blood in the LV
The mixing dilutes the amount of oxygen in the blood leading to less oxygen available in blood and decreased oxygen saturation
Less oxygen is delivered to the brain
S/s occurs like rapid breathing Cyanotic lips,tongue and fingers fainting
CLINICAL MANIFESTATION Cyanosis is an important sign of tetralogy of Fallot. Cyanosis is a bluish tint to the skin, lips, and fingernails. Low oxygen levels in the blood cause cyanosis. Babies who have unrepaired tetralogy of Fallot sometimes have "tet spells." These spells happen in response to an activity like crying or having a bowel movement. A TET spell occurs when the oxygen level in the blood suddenly drops. This causes the baby to become very blue. The baby also may: Have a hard time breathing Become very tired and limp Not respond to a parent's voice or touch Become very fussy Pass out In years past, when tetralogy of Fallot wasn't treated in infancy, children would get very tired during exercise and could faint. Now, doctors repair tetralogy of Fallot in infancy to prevent these symptoms. Another common sign of tetralogy of Fallot is a heart murmur. A heart murmur is an extra or unusual sound that doctors might hear while listening to the heart. Other signs Your baby may have other signs, such as: - Cool and clammy skin - Pale skin color - Poor feeding because the baby tires easily while nursing - Poor weight gain
Digital clubbing with cyanotic nail beds in an adult with tetralogy of Fallot
MEDICAL MANAGEMENT Emergency management of tet spells Prior to corrective surgery, children with tetralogy of Fallot may be prone to consequential acute hypoxia (tet spells), characterized by sudden cyanosis and syncope. These may be treated with beta-blockers such as propranolol, but acute episodes may require rapid intervention with morphine to reduce ventilatory drive and a vasopressor such as epinephrine, phenylephrine, ornorepinephrine to increase blood pressure. Oxygen (100%) is effective in treating spells because it is a potent pulmonary vasodilator and systemic vasoconstrictor. This allows more blood flow to the lungs. There are also simple procedures such as squatting and the knee chest position which increases aortic wave reflection, increasing pressure on the left side of the heart, decreasing the right to left shunt thus decreasing the amount of deoxygenated blood entering the systemic circulation. Palliative surgery The condition was initially thought untreatable until surgeon Alfred Blalock, cardiologist Helen B. Taussig, and lab assistant Vivien Thomasat Johns Hopkins University developed a palliative surgical procedure, which involved forming an anastomosis between the subclavian artery and the pulmonary artery (See movie "Something the Lord Made"). It was actually Helen Taussig who convinced Alfred Blalock that the shunt was going to work. This redirected a large portion of the partially oxygenated blood leaving the heart for the body into the lungs, increasing flow through the pulmonary circuit, and greatly relieving symptoms in patients. The first BlalockThomas-Taussig shunt surgery was performed on 15-month old Eileen Saxon on November 29, 1944 with dramatic results. The Potts shunt and the Waterston-Cooley shunt are other shunt procedures which were developed for the same purpose. These are no longer used. Currently, Blalock-Thomas-Taussig shunts are not normally performed on infants with TOF except for severe variants such as TOF withpulmonary atresia (pseudotruncus arteriosus). Total surgical repair The Blalock-Thomas-Taussig procedure, initially the only surgical treatment available for Tetralogy of Fallot, was palliative but not curative. The first total repair of Tetralogy of Fallot was done by a team led by C. Walton Lillehei at the University of Minnesota in 1954 on a 11year-old boy. Total repair on infants has had success from 1981, with research indicating that it has a comparatively low mortality rate. Total repair of Tetralogy of Fallot initially carried a high mortality risk. This risk has gone down steadily over the years. Surgery is now often carried out in infants one year of age or younger with less than 5% perioperative mortality. The open-heart surgery is designed (1) to relieve the right ventricular outflow tract stenosis by careful resection of muscle and (2) to repair the VSD with a Gore-Tex patch or a homograft. Additional reparative or reconstructive surgery may be done on patients as required by their particular cardiac anatomy.
Complete Intracardiac Repair Surgery to repair tetralogy of Fallot improves blood flow to the lungs. Surgery also ensures that oxygen-rich and oxygen-poor blood flow to the right places. The surgeon will: Widen the narrowed pulmonary blood vessels. The pulmonary valve is widened or replaced. Also, the passage from the right ventricle to the pulmonary artery is enlarged. These procedures improve blood flow to the lungs. This allows the blood to get enough oxygen to meet the body's needs. Repair the ventricular septal defect (VSD). A patch is used to cover the hole in the septum. This patch stops oxygen-rich and oxygen-poor blood from mixing between the ventricles. Fixing these two defects resolves problems caused by the other two defects. When the right ventricle no longer has to work so hard to pump blood to the lungs, it will return to a normal thickness. Fixing the VSD means that only oxygen-rich blood will flow out of the left ventricle into the aorta. The incision (cut) that the surgeon makes to reach the heart usually heals in about 6 weeks. The surgeon or a hospital staff member will explain when it's okay to give your baby a bath, pick him or her up under the arms, and take your baby for regular shots (immunizations). Diagnostic Tests and Procedures Your child's doctor may recommend several tests to diagnose tetralogy of Fallot. These tests can provide information about the four heart defects that occur in tetralogy of Fallot and how serious they are. Echocardiography Echocardiography (echo) is a painless test that uses sound waves to create a moving picture of the heart. During the test, the sound waves (called ultrasound) bounce off the structures of the heart. A computer converts the sound waves into pictures on a screen. Echo allows the doctor to clearly see any problem with the way the heart is formed or the way it's working. Echo is an important test for diagnosing tetralogy of Fallot because it shows the four heart defects and how the heart is responding to them. This test helps the cardiologist decide when to repair the defects and what type of surgery to use. Echo also is used to check a child's condition over time, after the defects have been repaired.
EKG (Electrocardiogram) An EKG is a simple, painless test that records the heart's electrical activity. The test shows how fast the heart is beating and its rhythm (steady or irregular). An EKG also records the strength and timing of electrical signals as they pass through the heart. This test can help the doctor find out whether your child's right ventricle is enlarged (ventricular hypertrophy). Chest X Ray A chest x ray is a painless test that creates pictures of the structures in the chest, such as the heart and lungs. This test can show whether the heart is enlarged or whether the lungs have extra blood flow or extra fluid, a sign of heart failure. Pulse Oximetry For this test, a small sensor is attached to a finger or toe (like an adhesive bandage). The sensor gives an estimate of how much oxygen is in the blood. Cardiac Catheterization During cardiac catheterization (KATH-eh-ter-ih-ZA-shun), a thin, flexible tube called a catheter is put into a vein in the arm, groin (upper thigh), or neck. The tube is threaded to the heart. Special dye is injected through the catheter into a blood vessel or one of the heart's chambers. The dye allows the doctor to see the flow of blood through the heart and blood vessels on an xray image. The doctor also can use cardiac catheterization to measure the pressure and oxygen level inside the heart chambers and blood vessels. This can help the doctor figure out whether blood is mixing between the two sides of the heart. NURSING MANAGEMENT Nursing diagnosis 1. 2. 3. 4. 5. Acute Pain Ineffective oxygenation of tissues Altered respiratory pattern Fatigue related to decrease oxygen Malnutrition less than body requirements
Nursing interventions bring the child's knees to the chest to increase the pressure of the left ventricle of the heart administer oxygen to the patient administer morphine to release the spasm of the pulmonary infundibulum.
PROGNOSIS Most cases can be corrected with surgery. Babies who have surgery usually do well. Ninety percent survive to adulthood and live active, healthy, and productive lives. Without surgery, death usually occurs by the time the person reaches age 20. Patients who have continued, severe leakiness of the pulmonary valve may need to have the valve replaced. Regular follow-up with a cardiologist to monitor for life-threatening arrhythmias (irregular heart rhythms) is recommended.
You might also like
- Medicine in Brief: Name the Disease in Haiku, Tanka and ArtFrom EverandMedicine in Brief: Name the Disease in Haiku, Tanka and ArtRating: 5 out of 5 stars5/5 (1)
- Tetralogy of FallotDocument28 pagesTetralogy of FallotArdyansyah Nasution, M.DNo ratings yet
- Tetralogy of Fallot Lesson PlanDocument25 pagesTetralogy of Fallot Lesson PlanUday Kumar100% (1)
- Sample Process Recording ColumnDocument6 pagesSample Process Recording ColumnLaurice Barron100% (1)
- Tetralogy of FallotDocument28 pagesTetralogy of FallotconcozNo ratings yet
- Tetralogy of FallotDocument8 pagesTetralogy of Fallotdecembergirl92No ratings yet
- Tetralogy of FallotDocument8 pagesTetralogy of FallotHillary Faye FernandezNo ratings yet
- Tetralogy of FallotDocument7 pagesTetralogy of FallotAshok Kumar JangirNo ratings yet
- Tetralogy of Fallot.......Document7 pagesTetralogy of Fallot.......Sam AlipioNo ratings yet
- Tetralogy of FallotDocument65 pagesTetralogy of FallotEndrati WidiastutiNo ratings yet
- Background TOFDocument2 pagesBackground TOFNyitnyit KunyitNo ratings yet
- Tetralogy of FallotDocument9 pagesTetralogy of FallotPaul MagbooNo ratings yet
- What Is Tetralogy of FallotDocument3 pagesWhat Is Tetralogy of FallotJyedenn PonceNo ratings yet
- What Is Tetralogy of FallotDocument2 pagesWhat Is Tetralogy of FallotJamaica Cruz San Pedro100% (1)
- Tetralogy of FallotDocument5 pagesTetralogy of FallotCharity OaniaNo ratings yet
- TOFDocument17 pagesTOFMaryel PascualNo ratings yet
- What Is Tetralogy of Fallot?: Congenital Heart DefectDocument3 pagesWhat Is Tetralogy of Fallot?: Congenital Heart DefectGlenn Asuncion PagaduanNo ratings yet
- Tetralogy of Fallot Is A Combination of Four Congenital AbnormalitiesDocument4 pagesTetralogy of Fallot Is A Combination of Four Congenital AbnormalitiesJoanne TolopiaNo ratings yet
- Tetralogy of Fallot: (A Congenital Heart Disease)Document7 pagesTetralogy of Fallot: (A Congenital Heart Disease)Sunil Kumar SahuNo ratings yet
- Tetralogy of FallotDocument7 pagesTetralogy of FallotWidelmark FarrelNo ratings yet
- Tetralogy of FallotDocument3 pagesTetralogy of FallotMiLody NajeNo ratings yet
- Tetralogy of Fallot: Arianna Jasminemabunga Bsn-2BDocument30 pagesTetralogy of Fallot: Arianna Jasminemabunga Bsn-2BArianna Jasmine MabungaNo ratings yet
- Tetralogy of Fallot - StatPearls - NCBI BookshelfDocument9 pagesTetralogy of Fallot - StatPearls - NCBI BookshelfElfrida AuliaNo ratings yet
- Magyawi Tetralogy of Fallot 1st PartDocument7 pagesMagyawi Tetralogy of Fallot 1st PartJoanna Bee Rose MagyawiNo ratings yet
- Tetralogy of FallotDocument33 pagesTetralogy of FallotjeenaejyNo ratings yet
- Tetralogy of FallotDocument6 pagesTetralogy of FallotArianna Jasmine MabungaNo ratings yet
- Tetralogy of Fallot SP B.inggrisDocument19 pagesTetralogy of Fallot SP B.inggrisSoraya VerinaNo ratings yet
- Presentation ON Tetrallogy of FallotDocument33 pagesPresentation ON Tetrallogy of Fallotvani reddyNo ratings yet
- Tetralogy of FallotDocument38 pagesTetralogy of FallotJohn Paul MedalloNo ratings yet
- Congenital AnomaliesDocument26 pagesCongenital AnomaliesMaria VisitacionNo ratings yet
- Congenital Heart Defec1Document3 pagesCongenital Heart Defec1Matt BaterzalNo ratings yet
- Cyanotic Spells Blue Spells in Tetralogy of Fallot - PIAG 214Document2 pagesCyanotic Spells Blue Spells in Tetralogy of Fallot - PIAG 214imil irsalNo ratings yet
- Tof Case ReportDocument11 pagesTof Case ReportPatrick DycocoNo ratings yet
- LESSON 3. Congenital Heart DefectsDocument9 pagesLESSON 3. Congenital Heart DefectsEdmarie P. TabacoldeNo ratings yet
- Odia Tetralogy of FallotDocument25 pagesOdia Tetralogy of Fallotvictorjonathan567No ratings yet
- Presented by DR Rahul D AgrawalDocument64 pagesPresented by DR Rahul D AgrawalRahul AgrawalNo ratings yet
- Congenital Heart DefectDocument2 pagesCongenital Heart DefectMatt BaterzalNo ratings yet
- Week 11 Post Test - Tof Group 29 12CDocument12 pagesWeek 11 Post Test - Tof Group 29 12CShane LuyNo ratings yet
- Tetralogy of FallotDocument31 pagesTetralogy of FallotDevipriya MajumderNo ratings yet
- Tetralogy of Fallot: Clinical Background AnatomyDocument12 pagesTetralogy of Fallot: Clinical Background AnatomyJoriza TamayoNo ratings yet
- Tetralogy of Fallot, Agarwala 2017Document5 pagesTetralogy of Fallot, Agarwala 2017rinayondaNo ratings yet
- Congenital Heart Diseases.Document86 pagesCongenital Heart Diseases.Salman KhanNo ratings yet
- Presentation ON Tetralogy of FallotDocument36 pagesPresentation ON Tetralogy of Fallotvani reddyNo ratings yet
- Tetralogy of FallotDocument31 pagesTetralogy of FallotAnditha Namira RS100% (1)
- Fallot TetralogyDocument20 pagesFallot Tetralogykgt88No ratings yet
- What Is Tetralogy of FallotDocument18 pagesWhat Is Tetralogy of FallotIyah Bu-ucanNo ratings yet
- Tetralogy of FallotDocument5 pagesTetralogy of FallotNoor FadhilaNo ratings yet
- Tetralogy of Fallot Report 1Document9 pagesTetralogy of Fallot Report 1Maricar EustaquioNo ratings yet
- Cardiovascular System Dr. Eman Badr 2020Document182 pagesCardiovascular System Dr. Eman Badr 2020Amina DinarNo ratings yet
- 12 - Semiotics of Cardiovascular Disorders. Semiotics of Congenital Hert Diseases in Children.Document30 pages12 - Semiotics of Cardiovascular Disorders. Semiotics of Congenital Hert Diseases in Children.Omowunmi KadriNo ratings yet
- Tetralogy of FallotDocument3 pagesTetralogy of FallotyasminNo ratings yet
- Pediatric Patent Ductus ArteriosusDocument12 pagesPediatric Patent Ductus Arteriosusabirami_murugesuNo ratings yet
- Tetralogy of FallotDocument5 pagesTetralogy of FallotSaloni MehtaNo ratings yet
- Tetralogy of Fallot: What Is It?Document3 pagesTetralogy of Fallot: What Is It?Anditha Namira RSNo ratings yet
- Pulmonary Stenosis: Narrowing of The Pulmonary Valve and Outflow Tract or Area BelowDocument1 pagePulmonary Stenosis: Narrowing of The Pulmonary Valve and Outflow Tract or Area BelowGlenn Asuncion PagaduanNo ratings yet
- Tetralogy of FallotDocument12 pagesTetralogy of Fallotneetuthakur100% (5)
- Tetralogy of FallotDocument10 pagesTetralogy of FallotanggiehardiyantiNo ratings yet
- Tiki Taka Notes Final PDFDocument104 pagesTiki Taka Notes Final PDFAditiSahak62No ratings yet
- Congenital Heart Diseases, A Simple Guide to these Medical ConditionsFrom EverandCongenital Heart Diseases, A Simple Guide to these Medical ConditionsNo ratings yet
- Csa SampleDocument12 pagesCsa SampleLaurice BarronNo ratings yet
- Colonoscopy Is Mandatory AfterDocument3 pagesColonoscopy Is Mandatory AfterLaurice BarronNo ratings yet
- Corruption in The Military Audited by Heidi MendozaDocument1 pageCorruption in The Military Audited by Heidi MendozaLaurice BarronNo ratings yet