Professional Documents
Culture Documents
Slides - 3
Slides - 3
Uploaded by
Rahul PandeyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Slides - 3
Slides - 3
Uploaded by
Rahul PandeyCopyright:
Available Formats
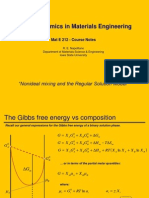
Fundamentals of Solidification
Outline
Introduction
Homogeneous nucleation
Heterogeneous nucleation
Growth and microstructure
Summary
Introduction
There are two types of solidification
Glass formation
Physical properties such as viscosity change
smoothly across the solidifying region
Phase transition
Some physical properties change abruptly,
such as viscosity, heat capacity
Introduction
Solidification by phase transition is
modelled as two stage
Nucleation
Homogeneous nucleation
Heterogeneous nucleation
Growth
Nucleation and Grain Growth
Nucleation;
Homogeneous nucleation: very pure metal, substantial
undercooling (0.2Tm)
Heterogeneous nucleation: nucleation agents (5C
undercooling)
Grain growth
Planar: pure metal
Dendritic: solid solution
Grain size
depends on number of nuclei and cooling rate.
Crystal Nucleation and Growth
Manufacturing Processes for Engineering Materials, by Serope Kalpakjian
7
Nucleation Rate
The rate at which nucleation of a new phase
occurs is critical to the prediction of phase
transformation behavior.
We will contrast homogeneous nucleation
(extremely rare!) with heterogeneous nucleation
(typical) rates.
Why study homogeneous nucleation? Useful
foundation and simplest to understand.
Bottom line: the quickest transformation wins!
8
Thermodynamics of nucleation
How should we understand nucleation?
The crucial point is to understand it as a balance
between the free energy available from the driving force,
and the energy consumed in creating new interface
(between parent and product phases). Once the rate of
change of free energy becomes negative, then an
embryo can grow.
Parallel to the Griffith analysis: once the rate of (free)
energy change becomes negative with crack length
increase, then the crack can grow without limit.
9
Nucleation paths
It is important to remember that the actual outcome is
always the process that leads most rapidly to the change for
which a (thermodynamic) driving force exists.
Anisotropy in the interfacial energy forces growing grains to
adopt anisotropic shapes in order to minimize high energy
orientations of the interface.
Anisotropy in growth rates has a similar effect.
Heterogeneous nucleation on surfaces, pre-existing
interfaces (grain boundaries), dislocations etc. is very
important.
Elastic energy plays a major role in constraining nucleation.
10
Homogeneous Nucleation
Assume that the new, product phase appears as spherical
particles.
Free energy released by transformation is proportional to
the volume.
Free energy consumed by creation of interface is
proportional to the surface area of particle and the
interfacial energy, g.
Net change in free energy per particle, G
r
:
G
r
= -4/3 r
3
G
V
+ 4r
2
g.
Differentiate to find the stationary point (at which the rate of
change of free energy turns negative).
11
Critical radius, free energy
d(G
r
) = 0 =
-4/ r*
2
G* + 8r*g.
From this we find the critical
radius and critical free energy.
r* = 2g/G
V
G* = 16g
3
/3G
V
2
Crucial difference from
solidification: the role of elastic
energy!
Not at G
r
=0!!!
12
Elastic energy
Why does elastic energy play such an important role in
solid state phase transformations?
Volume changes on transformation of order a few % are
typical. Elastic energy is symmetric: net (hydrostatic)
tension or compression leads to an increase in elastic
energy. This elastic energy cost for creation of a new
phase, G
S
(= Ee
2
/2), must be subtracted in proportion
to the volume of new phase.
G
r
= -4/3 r
3
(G
V
- G
S
) + 4r
2
g.
d(G
r
) = 0 = -4 r
2
G* + 8r*g.
r* = 2g / (G
V
- G
S
);
G* = 16g
3
/ 3(G
V
- G
S
)
2
.
Homogeneous nucleation
r
r
Homogeneous nucleation
No preferred nucleation sites
Spontaneous
Random
Those of preferred sites
Boundary
Surface
Inclusion,
Local free energy change
1. Liquid to solid 2. Interface
Single nucleus
Critical radius
0 / = A dr G d
( )
S L
SL
G G
r
=
o 2
*
( )
2
3
3
16
*
S L
SL
G G
G
= A
o
t
(G
L
-G
S
) vs. supercooling
Free energy density vs. temperature
liquid
solid
temperature
F
r
e
e
e
n
e
r
g
y
d
e
n
s
i
t
y
19
Homogeneous Nucleation: examples
Two examples of homogeneous nucleation in the solid
state are known.
1) Cu with 1-3% Co can be heat treated to precipitate Co
homogeneously. We will examine this case in a homework
aimed at predicting TTT diagrams.
2) Ni superalloys will precipitate Ni
3
Al homogeneously at
small undercoolings because of the small lattice misfit and
small interfacial energy.
Why only these cases? Small interfacial energy, and small
elastic energy difference.
Everything else: heterogeneous!
20
Elastic Anisotropy
Remember that most crystalline solids are elastically
anisotropic: this means that the shape of a new phase is
likely to be anisotropic.
If either the parent or the product phase is more compliant
in a particular direction, larger dimensions parallel to this
direction will be favored over stiffer directions. This is
offset by the interfacial energy term which must increase
as the surface-to-volume ratio increases.
Example: Guinier-Preston zones in the Al-Cu system,
which are platelets on {100}.
21
Interfacial Energy
Even in solidification, the anisotropy of the interfacial
energy matters. The energy of the solid-liquid interface
varies depending on which crystallographic surface is
involved. {111} surfaces tend to have the lowest energy in
fcc metals.
In solid state precipitation, the anisotropy of the interface
matters even more! This is because there are two
crystalline surfaces involved in the interface. If the crystal
structures are different (often the case) then low energy
interfaces require good atomic matching between the two
planes. Sometimes this results from combining close-
packed interfaces.
22
Nucleation rate
To estimate the nucleation rate we need to know the population
density of embryos of the critical size and the rate at which such
embryos are formed.
Population (concentration) of critical embryos, C*, is given by a
Boltzmann factor, where C
0
is the number of atoms per unit
volume:
C* = C
0
exp -(G*/kT)
The rate at which a critical embryo is formed, f, depends on the
migration of atoms, i.e. diffusion, which is again given by a
Boltzmann factor, where G
bulk
is the activation energy for (bulk)
diffusion (G
m
in P&E), and w is of the same order as the atomic
jump frequency:
f = w exp -(G
bulk
/kT).
23
Nucleation rates, contd.
Based on this approach,
we can now understand
the extremely strong
dependence of nucleation
rate on under cooling.
Note that the net effect of
elastic energy is to offset
(decrease) the equilibrium
transformation
temperature.
24
Effect of undercooling
The effect of undercooling on the nucleation rate is drastic,
because of the non-linear relation between the two quantities.
By incorporating the previous expression into the nucleation rate
we obtain the following:
C* = C
0
exp -(G*/kT) =
Finally the nucleation
rate is the product
of C* and f:
N = f C*
C* = C
0
exp
16t
3
3 AG
V
AG
S
( )
2
kT
`
)
= C
0
exp
16t
3
3
AHAT
T
e
AG
S
|
\
|
.
|
|
2
kT
`
)
25
Nucleation Rate
The combined equations are as follows.
The nucleation rate is the product of
C* and f. Note that the product of w and C
0
is a large
number because w is of the order of the atomic vibration
frequency, and C
0
is the number of atoms per unit volume.
N = f C *= eexp
AG
bulk
kT
`
)
C
0
exp
16t
3
3
AHAT
T
e
AG
S
|
\
|
.
|
2
kT
`
)
Heterogeneous nucleation
Nucleation site
Mold walls
Inclusion
Interface
Surface
Impurity
27
Heterogeneous Nucleation
Heterogeneous nucleation must occur on some substrate:
grain boundaries
triple junctions
dislocations
(existing) second phase particles
Consider a grain boundary: why is it effective? Answer:
by forming on a grain boundary, an embryo can offset its
cost in interfacial energy by eliminating some grain
boundary area.
Liquid
Inclusion
Nucleus u o
IL
o
NL
o
IN
R
r
h
a
u
Heterogeneous nucleation
29
Grain boundary nucleation
The semi-angle, q = cos
-1
g
aa
/2g
ab
As for solidification, the radius of the spherical caps
depends only on the interfacial energy, so:
r* = 2g
ab
/(G
V
-G
S
)
but a shape factor modifies the critical free energy:
G* = 16g
ab
3
/3(G
V
-G
S
)
2
S(q)
= 16g
ab
3
/3(G
V
-G
S
)
2
0.5(2 + cosq)(1 - cosq)
2
o|
|
o
oo
u
Grain
boundary
in alpha
30
Other heterogeneous sites
Other sites for
heterogeneous nucleation
have been listed.
For the same contact angle,
grain corners (quadruple
points) are more effective
than grain edges (triple
lines), which are more
effective than grain
boundaries.
31
Heterogeneous nucleation rate
The rate of heterogeneous nucleation, N
het
, is described by a
very similar equation as previously described for
homogeneous nucleation, N
homo
. The critical difference is in
the critical free energy, G*, and the density of sites, C
1
.
Homogeneous:
N
homo
= e exp -(G
bulk
/kT) C
0
exp -(G
homo
*/kT)
Heterogeneous:
N
het
= e exp -(G
bulk
/kT) C
1
exp -(G
het
*/kT)
For grain boundary nucleation, for example, the ratio of site
densities, C
1
/C
0
= o/D, where D is the grain size, and o is the
boundary thickness.
Local free energy change
( )
SL L S before after
A G G V G G G o + = = A
( )
SL S L
r G G r G o t
t
2 3
4
3
4
+ = A
Spherical nucleus:
Thermodynamic barriers
Heterogeneous
nucleation barrier
Homogeneous
nucleation barrier
System free energy
Ideal solution: Particle of different sizes
n
i
particles with each contains i atoms
n particles with each contains 1 atom
S T G n G
i c
A A = A
(
|
|
.
|
\
|
+
+
|
|
.
|
\
|
+
= A
i i
i
i
n n
n
n
n n
n
n k S ln ln
Number of nuclei
At equilibrium
0 / = c A c
i c
n G
|
|
.
|
\
|
+
=
A
i
i
n n
n
kT
G
ln
i
n n >>
|
.
|
\
|
A
=
kT
G
n n
i
exp
|
.
|
\
|
A
=
kT
G
n n
i
*
exp *
when
Number of nuclei
Boltzmann formula:
Critical nuclei:
Inoculating agents
Small interface energy
Similar crystal structure
Similar lattice distance
Same physical properties
Same chemical properties
Casting refinement
Adding inoculating agents
Overheat might melt the agents
Surface refinement
Coat agents on mold walls
Pattern induced solidification
Growth and microstructure
T. F. Brower and M.C. Flemings, Trans. AIME, 239, 1620 (1967)
H.B. Dong and P.D. Lee, Acta Mater. 53 (2005) 659
Growth and microstructure
Outer chilled zones
Outer chilled zones
Outer chilled zones
Outer chilled zones
Pure metals: Formation of shell because temperature
gradient is the key factor in grain growth.
Outer chilled zones
re-melted?
Pouring temperature
survived?
Microstructure of ingot
Chilled zone
Fine equiaxed grains.
Pure substance: Continuous shell.
Solution: Particles
Particles flushed away from wall into the
central
Re-melted
Survived nucleus
Intermediate columnar zone
Columnar grains grows
The grain is overtaken by neighbors.
Intermediate columnar zone
Growth and overtaken
Intermediate columnar zone
Columnar growth blocked
Central equiaxed zone
Equiaxed grain
Nucleation:
Supercooling
Falling particles
Dendrite fragments
Elevated pouring
temperature:
Larger equiaxed
grains
More columnar zone
Anisotropic properties
Magnetic materials
Turbo blade.
More equiaxed zone
Isotropic properties
Less segregation
Structure and properties
You might also like
- ElectroplatingDocument18 pagesElectroplatingRahul Pandey100% (2)
- AY2010 CE2134 Hydraulics E04 First Law of Thermodynamics Frictional Losses in Pipe FlowsDocument18 pagesAY2010 CE2134 Hydraulics E04 First Law of Thermodynamics Frictional Losses in Pipe FlowsEmily ShumNo ratings yet
- ZrO2-CaO Phase DiagramDocument1 pageZrO2-CaO Phase DiagramJohn Joseph100% (2)
- Quiz 3 SolutionsDocument4 pagesQuiz 3 SolutionsM.USMAN BIN AHMED0% (1)
- Material Science Assignment Solution Class Activity Crystal Structure 1Document4 pagesMaterial Science Assignment Solution Class Activity Crystal Structure 1yudhispokemon100% (1)
- Hard Chromium ElectroplatingDocument14 pagesHard Chromium ElectroplatingRahul PandeyNo ratings yet
- Solidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalDocument62 pagesSolidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalIndranil Bhattacharyya100% (1)
- Rolling - Nptel PDFDocument10 pagesRolling - Nptel PDFAmit RoyNo ratings yet
- Introduction To Electrometallurgy SyllabusDocument3 pagesIntroduction To Electrometallurgy SyllabusSalem GarrabNo ratings yet
- Material Science Cht04 and Cht08Document43 pagesMaterial Science Cht04 and Cht08Arnaldo Bester67% (3)
- MM 231 Phase Equilibria and Microstructures: Course Instructor: TauheedDocument151 pagesMM 231 Phase Equilibria and Microstructures: Course Instructor: TauheedGikiTopiNo ratings yet
- Electrometallurgy PDFDocument34 pagesElectrometallurgy PDFJeremy PutraNo ratings yet
- Dokumen - Tips Fundamentals of Modern Manufacturing 4th Solution ManualDocument25 pagesDokumen - Tips Fundamentals of Modern Manufacturing 4th Solution ManualNguyễn Đức MạnhNo ratings yet
- Fuels and CombustionDocument12 pagesFuels and CombustionAbhishek PrasadNo ratings yet
- Phase Diagrams Material ScienceDocument46 pagesPhase Diagrams Material ScienceSabir Ali100% (1)
- Ferrite Processing: Powder Preparation-Raw Materials SelectionDocument66 pagesFerrite Processing: Powder Preparation-Raw Materials Selection吳尚謙No ratings yet
- Chapter 10 Thermal Processing of Metal AlloysDocument44 pagesChapter 10 Thermal Processing of Metal Alloyssyed izzuddin alhadyNo ratings yet
- True Centrifugal CastingDocument2 pagesTrue Centrifugal CastingSaiful Zakwan50% (2)
- Material Science and MetallurgyDocument43 pagesMaterial Science and MetallurgySanjay Kumar SinghNo ratings yet
- Chapter 3 MFG IIDocument145 pagesChapter 3 MFG IITiliksew Wudie Assabe100% (1)
- Momentum TransferDocument40 pagesMomentum TransferDecien Dee Ferraren-De CagalitanNo ratings yet
- General Layout of Modern Steam Power PlantDocument15 pagesGeneral Layout of Modern Steam Power PlanttabishkhanaligNo ratings yet
- CH 07 Ternary PhaseDocument53 pagesCH 07 Ternary PhaseJhonnBeikerAnccasiLacho0% (1)
- Thermal Characterization Techniques2019 - HandoutsDocument8 pagesThermal Characterization Techniques2019 - HandoutsMuhammad OsamaNo ratings yet
- Phase TransformationDocument50 pagesPhase TransformationJitenderNo ratings yet
- Materials Science and Engineering by Callister Chapter 1 ReviewerDocument3 pagesMaterials Science and Engineering by Callister Chapter 1 ReviewerTy ztickNo ratings yet
- Ball MillDocument4 pagesBall MillGM MalikNo ratings yet
- Tutorial: GEARS II: Spur Gears - Force AnalysisDocument14 pagesTutorial: GEARS II: Spur Gears - Force AnalysisNdivhuwo NdivhuwoNo ratings yet
- ME8391 ETD by WWW - Learnengineering.inDocument167 pagesME8391 ETD by WWW - Learnengineering.inERRAMESH1989No ratings yet
- 1-5-6 SprueDocument45 pages1-5-6 Sprueuday kiran thagirchiNo ratings yet
- Solid Modeling Techniques: Half Spaces Boundary Representation (B-Rep)Document21 pagesSolid Modeling Techniques: Half Spaces Boundary Representation (B-Rep)rahul.yerrawarNo ratings yet
- HT Notes For ESEDocument25 pagesHT Notes For ESEM BhurleNo ratings yet
- ME8492 Kinematics of Machinery Notes 1 by WWW - Studymaterialz.inDocument123 pagesME8492 Kinematics of Machinery Notes 1 by WWW - Studymaterialz.inyuvaraj gopalNo ratings yet
- SN1 ReactionDocument17 pagesSN1 Reactionsp_putulNo ratings yet
- Bridgman Method - Bridgman Furnace - Crystal GrowthDocument4 pagesBridgman Method - Bridgman Furnace - Crystal GrowthImanKazemianNo ratings yet
- DomDocument3 pagesDomds_shivaNo ratings yet
- Crystal StructureDocument14 pagesCrystal StructureMahesh Lohith K.S100% (4)
- MM PDF Ia1Document109 pagesMM PDF Ia1M.41Mohd AnasNo ratings yet
- Heat Transfer Lecture Notes 2 (2016)Document10 pagesHeat Transfer Lecture Notes 2 (2016)Michael Belmonte UrdanetaNo ratings yet
- Hydrogen Production Using ElectrolysisDocument15 pagesHydrogen Production Using ElectrolysisInda RobayaniNo ratings yet
- Electochemistry PDFDocument29 pagesElectochemistry PDFAnshu KarmacharyaNo ratings yet
- 04 Crystal DefectsDocument40 pages04 Crystal Defectsnitesh_n2840No ratings yet
- AY2018-2019 - Exercise On Welding ProcessesDocument27 pagesAY2018-2019 - Exercise On Welding ProcessesCristina Barbu100% (1)
- TD-lecture NotesDocument135 pagesTD-lecture NotesAbdelkader Faklani Dou100% (1)
- Chapter 19-Bulk Deformation Processes IDocument67 pagesChapter 19-Bulk Deformation Processes IMohsin AliNo ratings yet
- Diffusion in Solids 3Rd Phase TransportDocument41 pagesDiffusion in Solids 3Rd Phase TransportgereNo ratings yet
- Chapter Five PDFDocument24 pagesChapter Five PDFعبدالله رعد حران 32No ratings yet
- HMT - 2 Marks PDFDocument29 pagesHMT - 2 Marks PDFFaiyu MechNo ratings yet
- Thermodynamic NotesDocument5 pagesThermodynamic NotesKarthick JyothieshwarNo ratings yet
- Molecular SymmetryDocument16 pagesMolecular SymmetryKirk Borromeo100% (1)
- Paschen-Back EffectDocument3 pagesPaschen-Back EffectSreedevi KrishnakumarNo ratings yet
- Gate QuesDocument5 pagesGate Quespsmonu54No ratings yet
- Engineering Thermodynamics: Experiment# 08 (Open Ended Lab)Document17 pagesEngineering Thermodynamics: Experiment# 08 (Open Ended Lab)kashmiri food and culture and bunkNo ratings yet
- 2151902Document49 pages2151902swarajNo ratings yet
- MESF5430 Note 3 For Students 2021Document17 pagesMESF5430 Note 3 For Students 2021KayNgNo ratings yet
- MIE270 Textbook Readings - Ch.11Document19 pagesMIE270 Textbook Readings - Ch.11sierra.millerNo ratings yet
- Paper Nature GosiaDocument4 pagesPaper Nature GosiaAna Paula da LuzNo ratings yet
- Introduction To SolidificationDocument30 pagesIntroduction To Solidificationgeraye0No ratings yet
- Structural Analysis of Nanomaterials: Lecture 05: Transformation of PhasesDocument30 pagesStructural Analysis of Nanomaterials: Lecture 05: Transformation of PhaseswinnieNo ratings yet
- MS Module 3Document16 pagesMS Module 3Affan KhanNo ratings yet
- Kuliah-6 MetFis2-Crystal InterfaceDocument59 pagesKuliah-6 MetFis2-Crystal InterfaceYusuf Bayu AjiNo ratings yet
- Kuliah-6 MetFis2-Crystal InterfaceDocument59 pagesKuliah-6 MetFis2-Crystal InterfaceYusuf Bayu AjiNo ratings yet
- Presentation ON SMS: Prepared By:Shashank Poddar Metallurgy (5 Sem)Document15 pagesPresentation ON SMS: Prepared By:Shashank Poddar Metallurgy (5 Sem)Rahul PandeyNo ratings yet
- A Presentation ON Vocational Training: Sandeep Pradhan 3203810039Document22 pagesA Presentation ON Vocational Training: Sandeep Pradhan 3203810039Rahul PandeyNo ratings yet
- A Report On Steel Melting ShopDocument18 pagesA Report On Steel Melting ShopRahul PandeyNo ratings yet
- Roll No 33Document39 pagesRoll No 33Rahul PandeyNo ratings yet
- Presentation On SMS: BY:-Ram Prasad Choudhary 3203810034Document19 pagesPresentation On SMS: BY:-Ram Prasad Choudhary 3203810034Rahul PandeyNo ratings yet
- Blast Furnac E: S.Megha Metallurgy SemesterDocument18 pagesBlast Furnac E: S.Megha Metallurgy SemesterRahul PandeyNo ratings yet
- Presentation ON Steel Melting Shop: Rupendra Naik 3203810037Document19 pagesPresentation ON Steel Melting Shop: Rupendra Naik 3203810037Rahul PandeyNo ratings yet
- Aditya Pratap Singh: Prepared ByDocument14 pagesAditya Pratap Singh: Prepared ByRahul PandeyNo ratings yet
- A Presentation On Dri-Ii in JSPL, Raigarh: By:-Harish Patel (Meta-5 Sem)Document14 pagesA Presentation On Dri-Ii in JSPL, Raigarh: By:-Harish Patel (Meta-5 Sem)Rahul PandeyNo ratings yet
- Summer Vocational Training Report: Bhilai Steel Plant, Sail ON "Mill Zone"Document26 pagesSummer Vocational Training Report: Bhilai Steel Plant, Sail ON "Mill Zone"Rahul PandeyNo ratings yet
- Roll No 59Document25 pagesRoll No 59Rahul PandeyNo ratings yet
- A Project Report On Summer Vocational Training in Bhilai Steel PlantDocument24 pagesA Project Report On Summer Vocational Training in Bhilai Steel PlantRahul PandeyNo ratings yet
- A Presentation On Dri-Ii: BY:-Vikash Ranjan Sharma Metallurgy - 5 SEM 3203810051Document15 pagesA Presentation On Dri-Ii: BY:-Vikash Ranjan Sharma Metallurgy - 5 SEM 3203810051Rahul PandeyNo ratings yet
- Regular SolutionsDocument19 pagesRegular SolutionsRahul PandeyNo ratings yet
- Presentation On Steel Melting Shop: BY: Dipti Dubey. Metallurgy 5 SEMDocument18 pagesPresentation On Steel Melting Shop: BY: Dipti Dubey. Metallurgy 5 SEMRahul PandeyNo ratings yet
- Arrhenius EqnDocument10 pagesArrhenius EqnRahul PandeyNo ratings yet
- Phase DiagramsDocument72 pagesPhase DiagramsRahul PandeyNo ratings yet
- Zinc Plating: Randhir Kumar Singh Asst Professor OpjitDocument16 pagesZinc Plating: Randhir Kumar Singh Asst Professor OpjitRahul PandeyNo ratings yet
- AnodizingDocument24 pagesAnodizingRahul Pandey100% (2)
- Corrosion MechanismsDocument64 pagesCorrosion MechanismsRahul PandeyNo ratings yet
- Tin PlatingDocument30 pagesTin PlatingRahul Pandey100% (3)
- Steel Melt Shop 2 Process .Document50 pagesSteel Melt Shop 2 Process .Rahul Pandey0% (2)
- Nickel ElectroplatingDocument28 pagesNickel ElectroplatingRahul Pandey100% (2)
- Perencanaan Pelat LantaiDocument16 pagesPerencanaan Pelat LantaiNelson AugustoneNo ratings yet
- Construction Material Lecture NoteDocument12 pagesConstruction Material Lecture NoteMohamedNo ratings yet
- Mnit Admission LetterDocument1 pageMnit Admission LetterAjaj AlamNo ratings yet
- What Is Nelco N5000-3032 BT PCBDocument5 pagesWhat Is Nelco N5000-3032 BT PCBjackNo ratings yet
- 19-GB 50030-91氧气站设计规范en PDFDocument34 pages19-GB 50030-91氧气站设计规范en PDFyyyy071220No ratings yet
- Click Here To Download IG Reels GuideDocument12 pagesClick Here To Download IG Reels GuidePampuliciNo ratings yet
- 2021 May Intake Advert Revised 4Document2 pages2021 May Intake Advert Revised 4douglas0% (1)
- 3800 PDFDocument202 pages3800 PDFRenny MataNo ratings yet
- Pompa Sirkulasi AP 156,3 LPM @60mDocument3 pagesPompa Sirkulasi AP 156,3 LPM @60mAndreas B KresnawanNo ratings yet
- Manual Conefor 26Document19 pagesManual Conefor 26J. Francisco Lavado ContadorNo ratings yet
- P 40 Color BrochureDocument4 pagesP 40 Color BrochureProject Sales CorpNo ratings yet
- 00-Saip-06 Pressure TestDocument4 pages00-Saip-06 Pressure TestUzair AhmadNo ratings yet
- Perhitungan TCDocument3 pagesPerhitungan TCManda PutraNo ratings yet
- Module 4 - Speed of SoundDocument16 pagesModule 4 - Speed of SoundLaiza GranaNo ratings yet
- ScanSnap Ix500 TroubleshootDocument58 pagesScanSnap Ix500 TroubleshootrajinbacaNo ratings yet
- FrictionDocument62 pagesFrictionchellamvNo ratings yet
- 2022 Msi-3460Document2 pages2022 Msi-3460Merced Alfonso Cepeda DíazNo ratings yet
- RR2009 - Generale-RrDocument39 pagesRR2009 - Generale-Rrhemant9315No ratings yet
- Sigma Marine Coatings Manual - Part79Document2 pagesSigma Marine Coatings Manual - Part79Tommy2020No ratings yet
- Failures in Subgrade: Properties of The Subgrade Soil Subgrade With Lower StabilityDocument2 pagesFailures in Subgrade: Properties of The Subgrade Soil Subgrade With Lower StabilityRajesh KhadkaNo ratings yet
- Product Safety Alert: 22 February 2021Document4 pagesProduct Safety Alert: 22 February 2021carlosorizabaNo ratings yet
- Clay.: There Are Six Main Soil TypesDocument4 pagesClay.: There Are Six Main Soil TypesSarah AyingNo ratings yet
- TD Mscaps 1 14.10.25 Doku API MyshortcartDocument20 pagesTD Mscaps 1 14.10.25 Doku API MyshortcartDA FI100% (1)
- Seismic: VvinuDocument528 pagesSeismic: VvinuLucas ScartonNo ratings yet
- NPS Drilling Safety Induction Rev 1Document108 pagesNPS Drilling Safety Induction Rev 1Sameer Parambath100% (1)
- Mechanical Operations (CHE-205) (Makeup) (EngineeringDuniya - Com)Document3 pagesMechanical Operations (CHE-205) (Makeup) (EngineeringDuniya - Com)Cester Avila Ducusin100% (1)
- Fispq-Senolith Uv Matt Lacquer - 07-11Document8 pagesFispq-Senolith Uv Matt Lacquer - 07-11Alexandre Antunes MateusNo ratings yet
- Snakebots: A Seminar Report ONDocument19 pagesSnakebots: A Seminar Report ONDibyaranjan SahooNo ratings yet
- Data Structures and Algorithms: (CS210/ESO207/ESO211)Document35 pagesData Structures and Algorithms: (CS210/ESO207/ESO211)Moazzam HussainNo ratings yet
- JINKO Mono PERC 390W-395W-400W-405W-410W - 24VDocument2 pagesJINKO Mono PERC 390W-395W-400W-405W-410W - 24VJose CaceresNo ratings yet